Methods for administering weight loss medications
a weight loss and composition technology, applied in the field of methods, can solve the problems of obesity being generally considered a psychological problem, unable to tolerate a full dosage of the indicated medication, and a global problem, so as to reduce the risk of adverse side effects, and reduce the effect of adverse side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiments
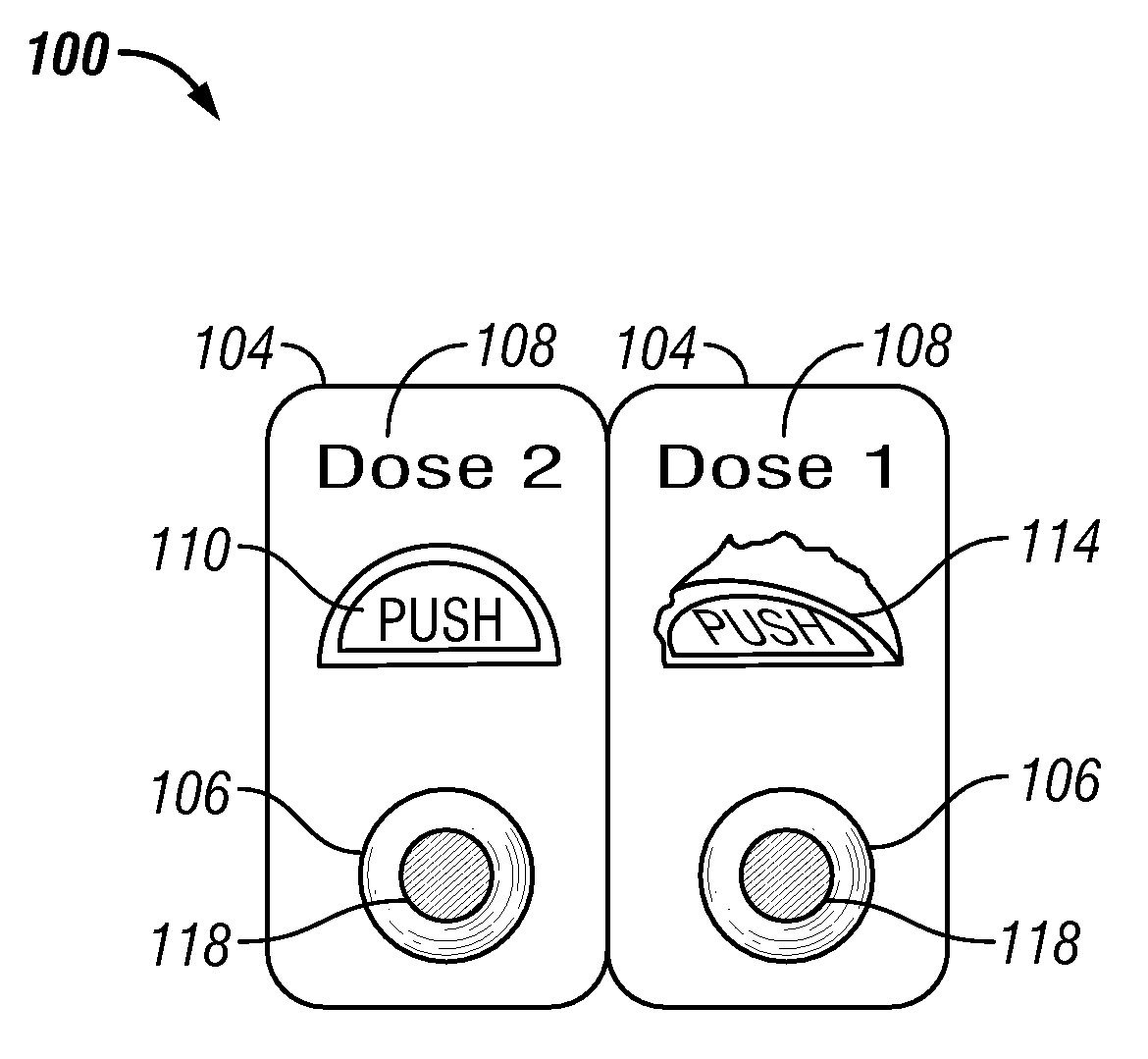
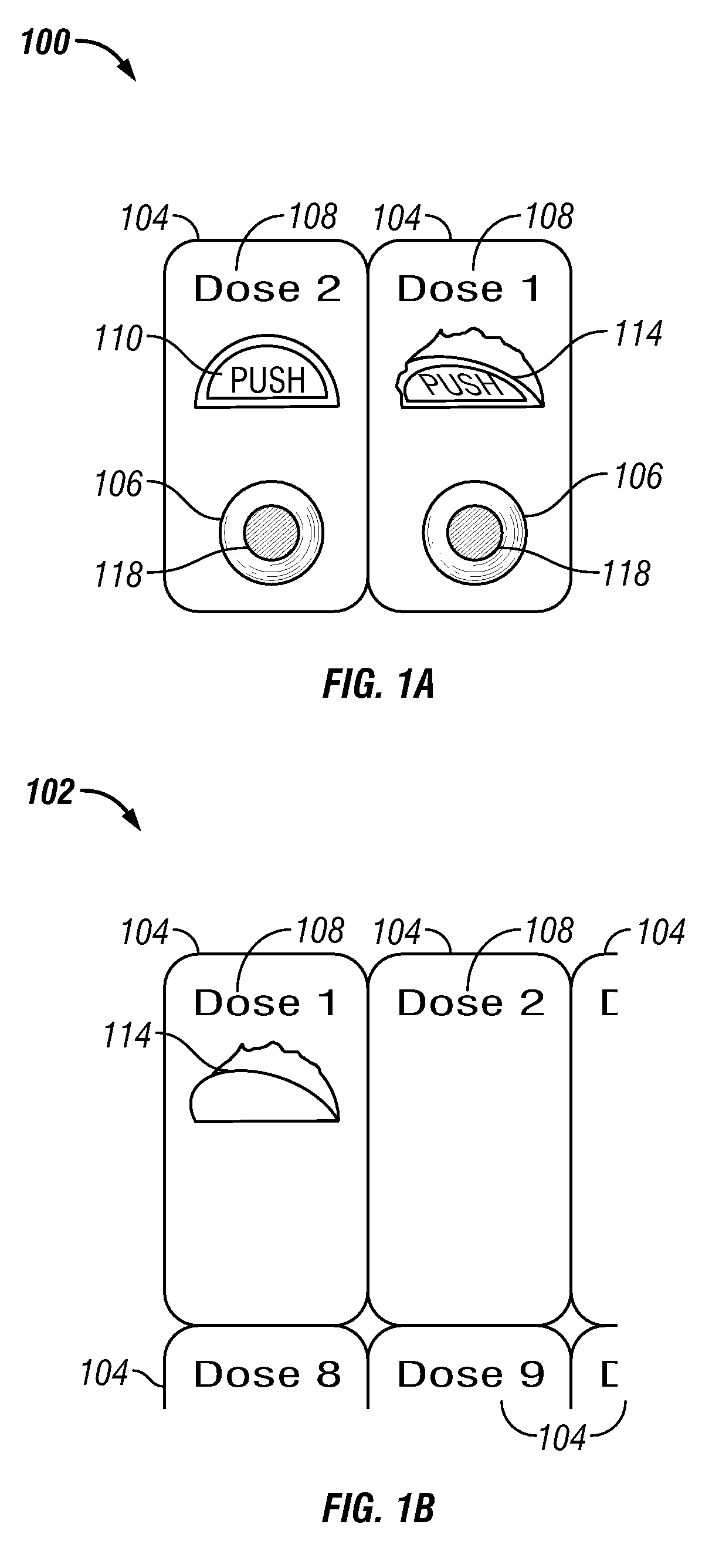
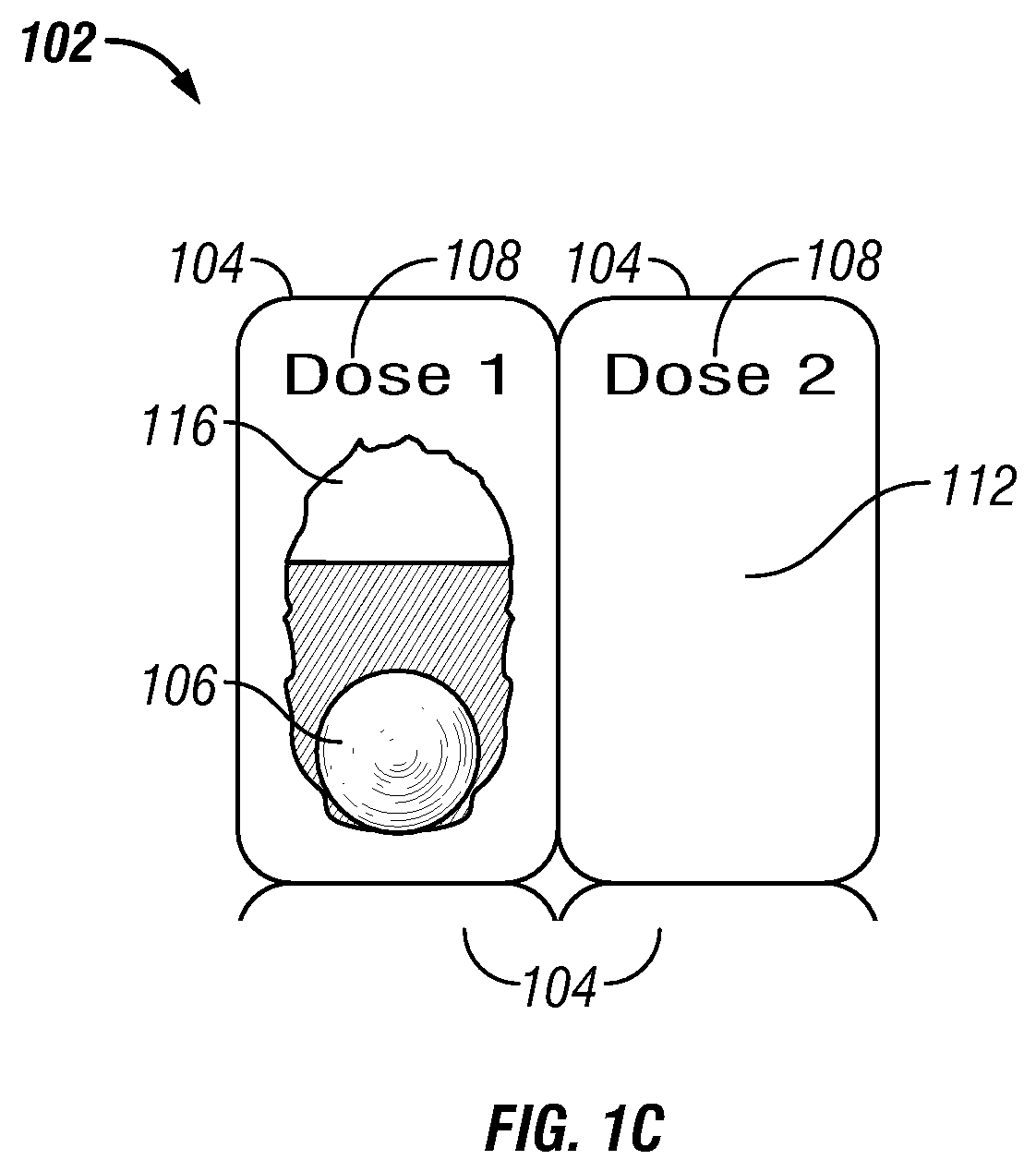
[0112]In one embodiment a method of treating a disease or condition comprises identifying a patient suffering from or at risk of said condition. In some embodiments the disease or condition is selected from the group consisting of affecting weight loss, suppressing appetite and. treating an obesity-related condition. In one embodiment the method comprises administering to a patient in need thereof a first dosage comprising a first drug and a second drug and administering a second dosage comprising the first drug and the second drug, wherein the second dosage comprises a different amount of the second drug than the first dosage.
[0113]In some embodiments the second dosage comprises a greater amount of the second drug than the amount of the second drug in the first dosage. In other embodiments the second dosage comprises a smaller amount of the second drug than the first dosage. In some embodiments the second dosage comprises a different amount of the first drug than the first dosage. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| physiological half life | aaaaa | aaaaa |
| physiological half life | aaaaa | aaaaa |
| physical | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com