Low-dose doxepin for treatment of sleep disorders in elderly patients
a technology of doxepin and elderly patients, which is applied in the direction of biocide, nervous disorders, drug compositions, etc., can solve the problems of sleep disorder that is often difficult to satisfactorily address or manage with available medications, and insomnia is a growing health problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Study to Evaluate Sleep Maintenance Effects of Three Dose Levels of Doxepin Hydrochloride (HCl) Relative to Placebo in Elderly Patients with Primary Insomnia
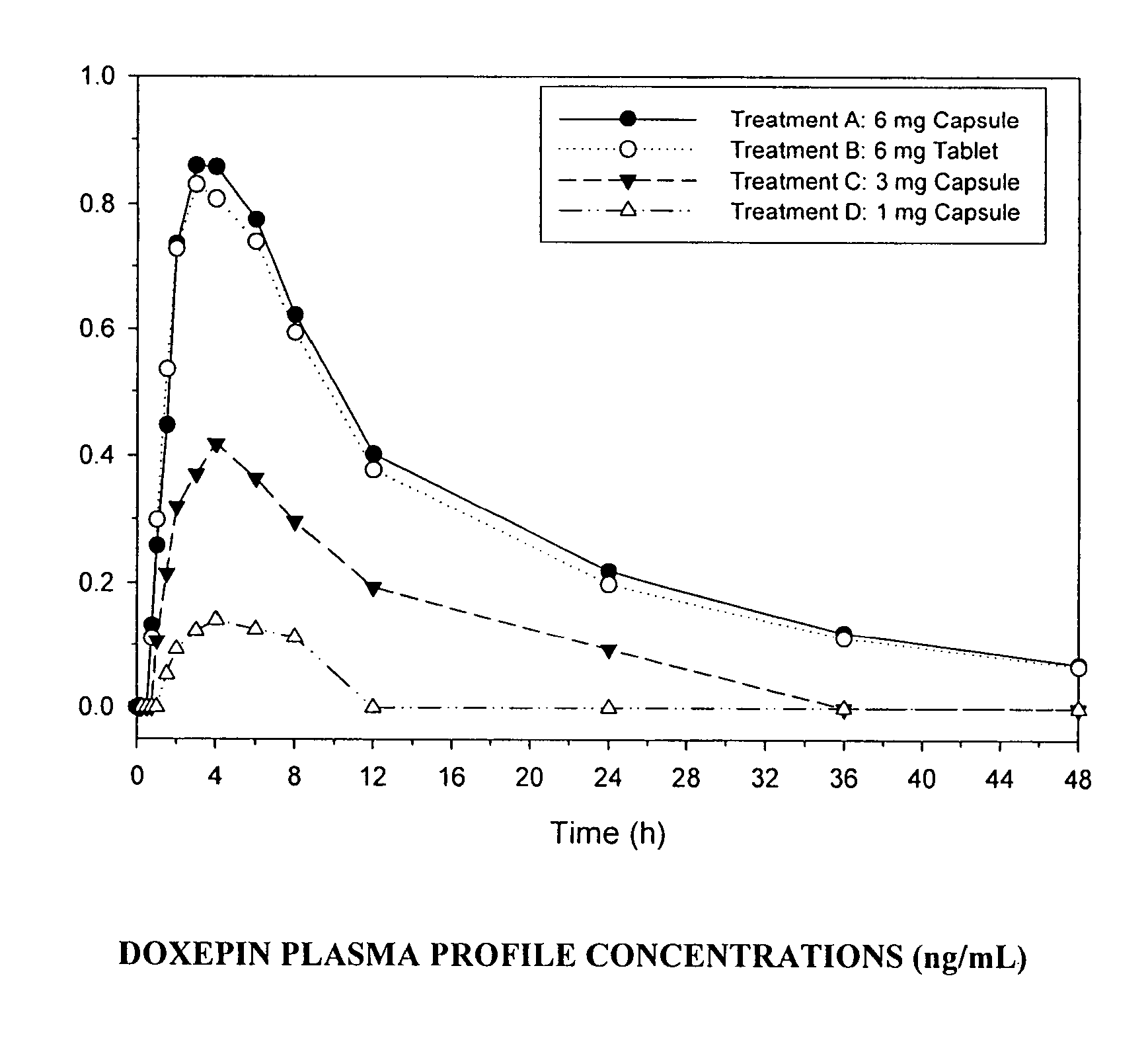
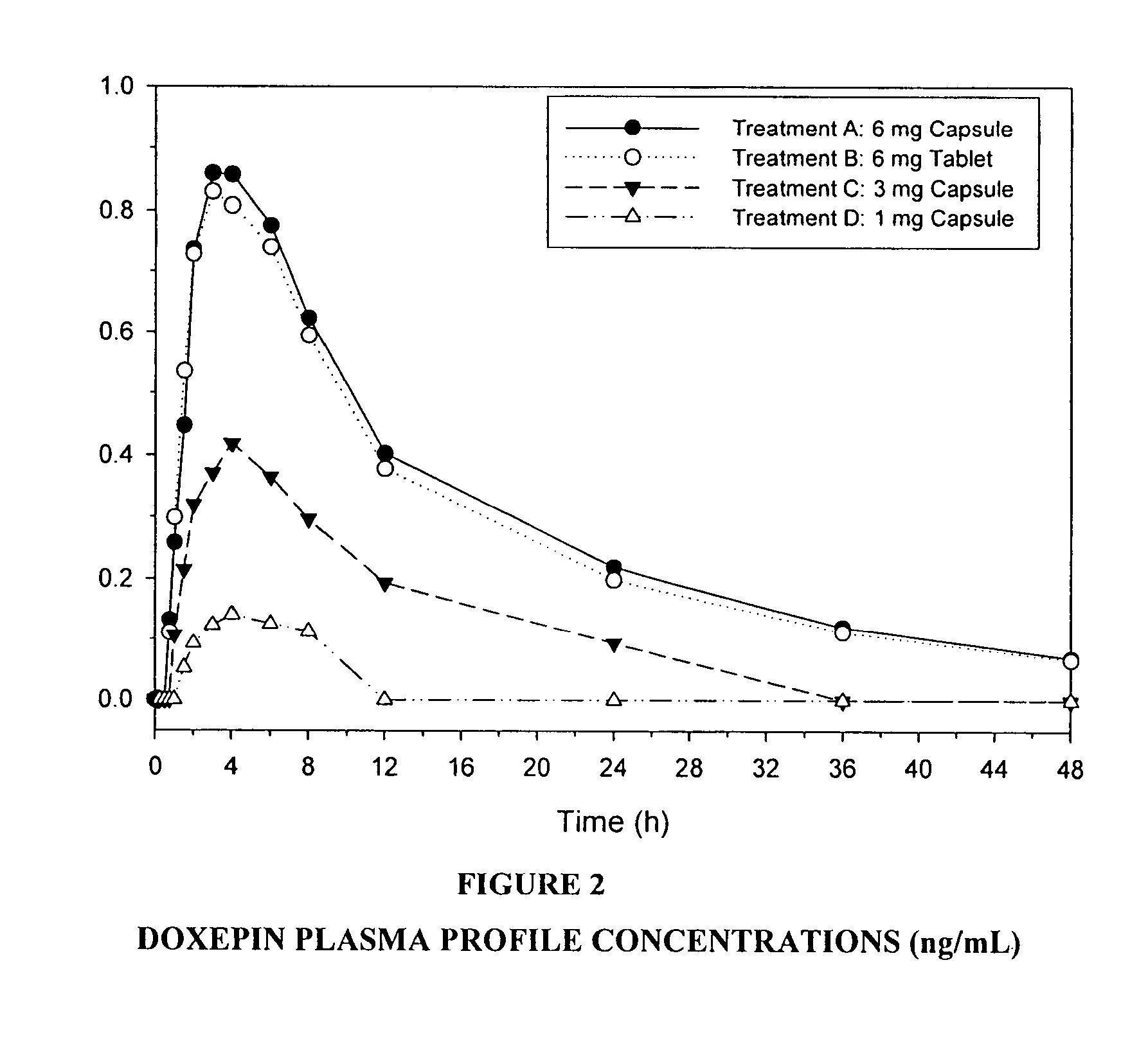
[0093]A randomized, multi-center, double-blind, placebo-controlled, four-period crossover, dose-response study was designed to assess the effects of doxepin (1 mg, 3 mg and 6 mg) compared with placebo in patients aged 65 years or older with primary sleep maintenance insomnia. Patients received a single-blind placebo for two consecutive nights during the PSG screening period, and double-blind study drug for two consecutive nights during each of the four treatment periods. Following each study drug administration, patients had 8 continuous hours of PSG recording in the sleep center. Patents were allowed to leave the sleep center during the day after each PSG assessment was complete. A 5- or 12-day study drug-free interval separated each PSG assessment visit. The duration of study participation per patient was approximately 7 to 11...
example 2
A Phase III, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Multicenter Study to Assess the Long Term Efficacy and Safety of Doxepin HCl in Primary Elderly Insomnia Patients with Sleep Maintenance Difficulties
[0105]A phase III, randomized, double-blind, placebo-controlled, parallel-group, multicenter study was conducted to assess the long term efficacy and safety of two dose levels of doxepin HCl, 1 mg and 3 mg, in primary elderly insomnia patients with sleep maintenance difficulties.
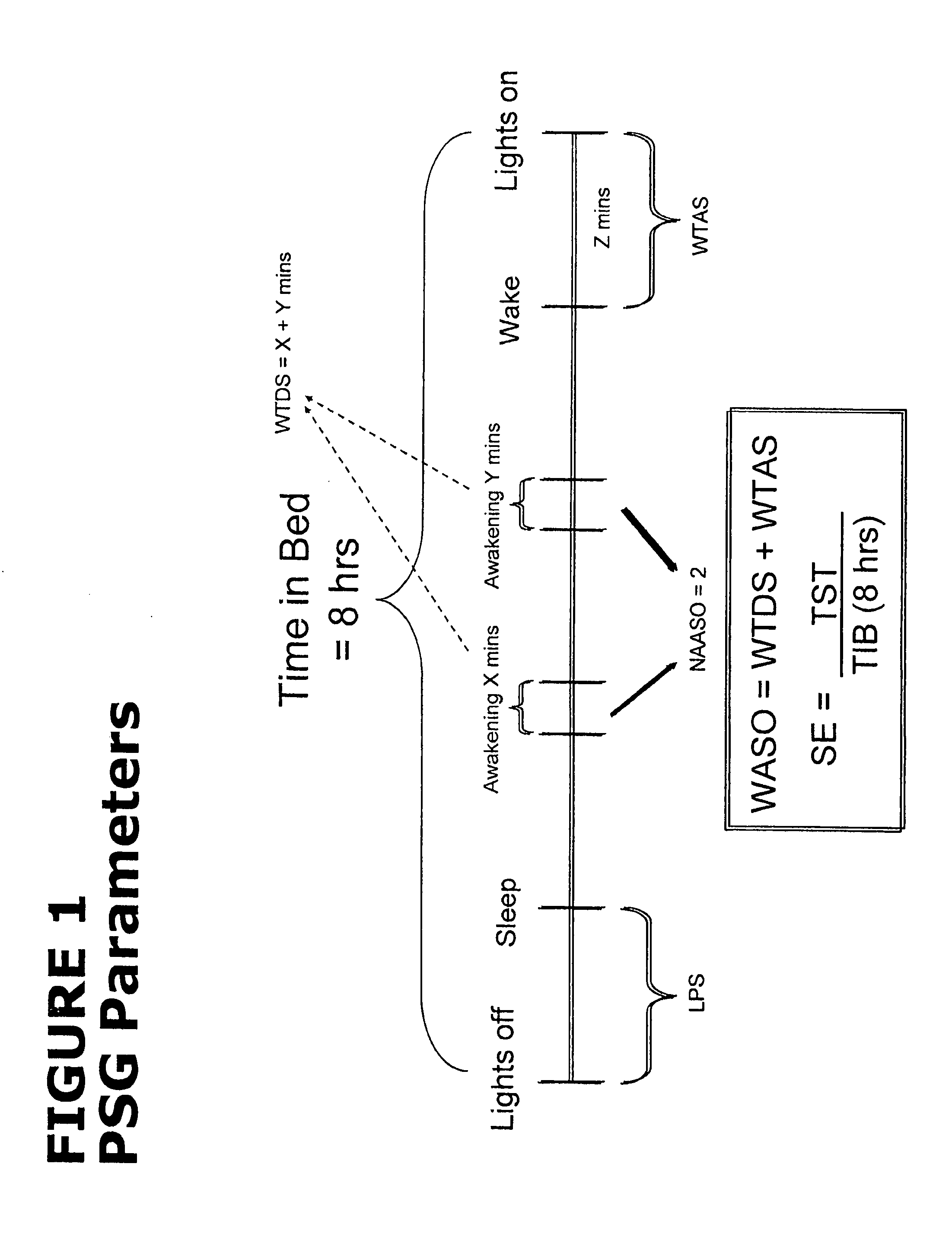
[0106]Subjects were females and males, 65 years of age or older, with at least a 3-month history of primary insomnia (as defined in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision), who reported experiencing at least 60 minutes of Wake After Sleep Onset (WASO), at least 30 minutes of Latency to Sleep Onset (LSO), and no more than 6.5 hours of Total Sleep Time (TST) on at least 4 of 7 consecutive nights prior to PSG Screening.
[0107]Doxepin 1 mg tabl...
example 3
Study to Evaluate Sleep Maintenance Effects of Three Dose Levels of Doxepin Hydrochloride (HCl) Relative to Placebo in Adult Patients (Ages 18-64) with Primary Insomnia
[0122]A randomized, multi-center, double-blind, placebo-controlled, four-period crossover, dose-response study was designed to assess the effects of doxepin (1 mg, 3 mg and 6 mg) compared with placebo in patients with primary sleep maintenance insomnia.
[0123]Patients received a single-blind placebo for two consecutive nights during the PSG screening period, and double-blind study drug for two consecutive nights during each of the four treatment periods. Following each study drug administration, patients had 8 continuous hours of PSG recording in the sleep center. Patents were allowed to leave the sleep center during the day after each PSG assessment was complete. A 5- or 12-day study drug-free interval separated each PSG assessment visit.
[0124]Patients who qualified for study entry, based on the screening PSG assessme...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com