Therapeutic Treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Treatment of a Patient Suffering from ADHD with and without an Appetite Stimulant
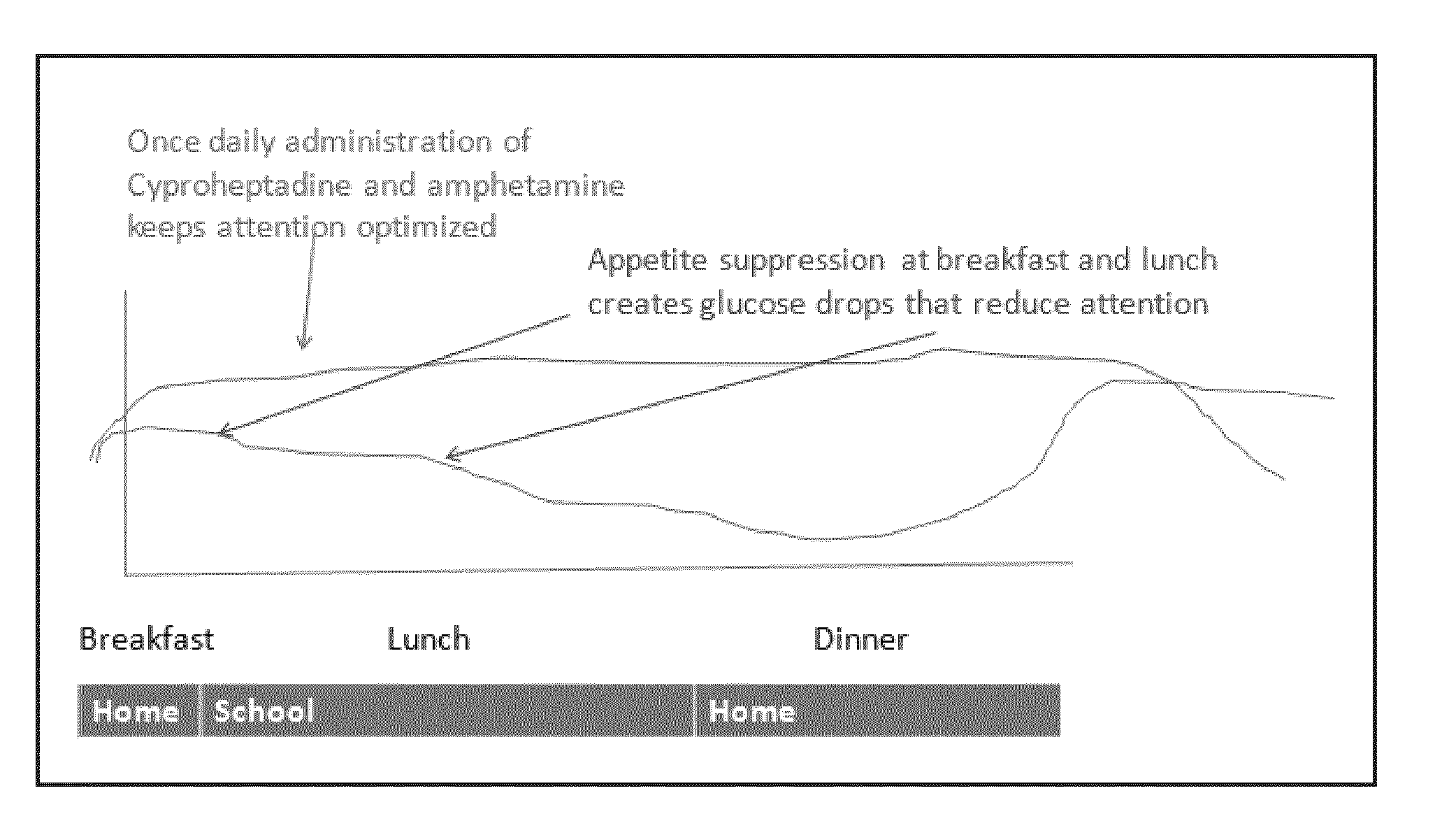
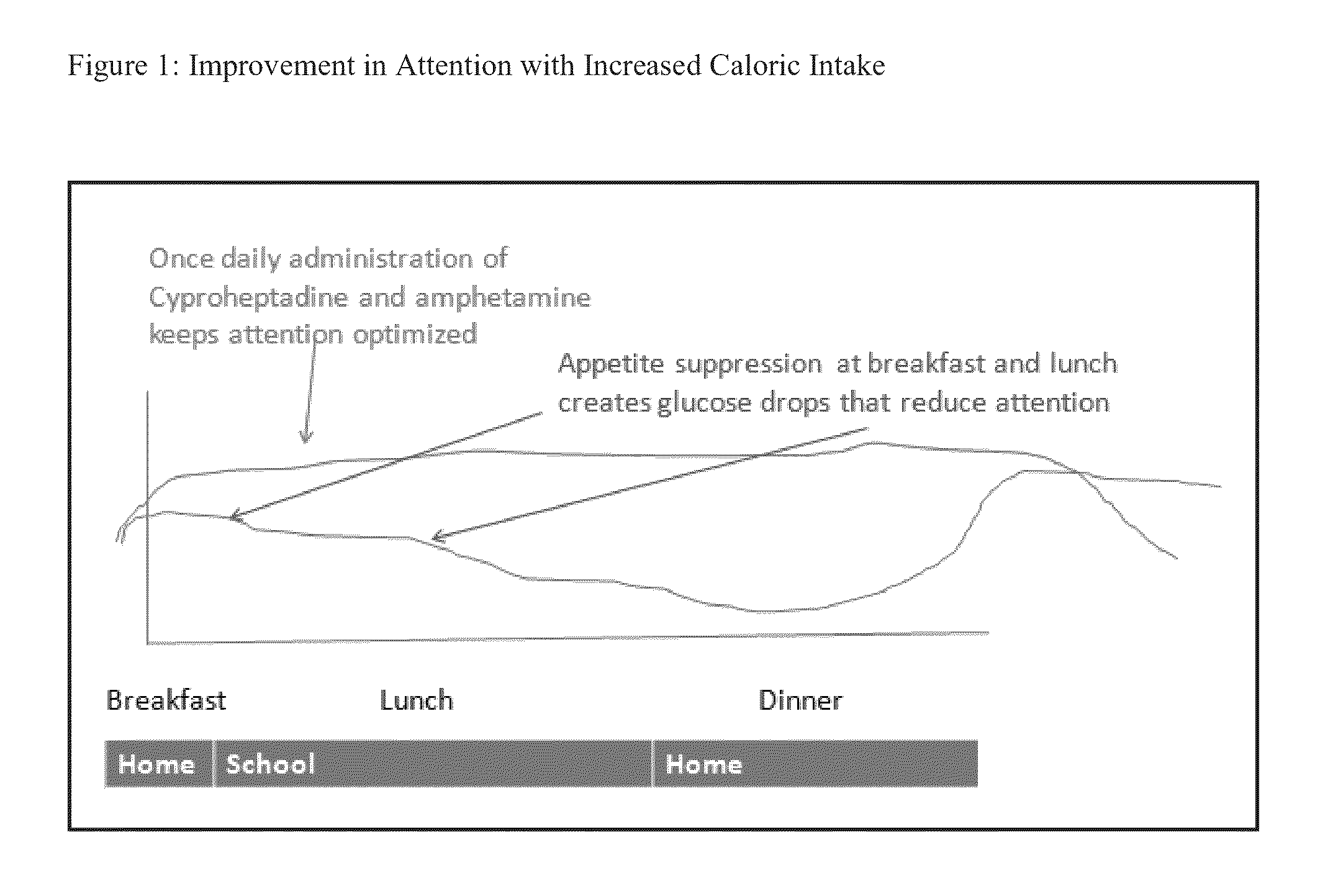
[0164]A patient suffering from ADHD is prescribed and administered an amphetamine to treat ADHD. The patient takes the amphetamine once a day in the morning or afternoon. Following administration, the patient suffers a loss of appetite and reduces their caloric intake. As a result, the glucose level measured in the blood drops as shown in FIG. 1 and the patient suffers a lack of attentiveness as measured by CGI-S and CGI-I. When the patient is administered an amphetamine and Cyproheptadine once a day either in the morning or afternoon, the patient's appetite resumes and the patient increases their caloric intake. As a result, the glucose level measured in the blood increases as shown in FIG. 1 and the patient's attentiveness recovers as measured by CGI-S and CGI-I. By taking the appetite stimulant during the time when the patient was awake, the patient increased their caloric intake during times when th...
example 2
Once a Day Periactin
[0165]Case study: A six-year and ten month old girl was presented by her parents for symptoms of attention deficit hyperactivity disorder. Her size at evaluation was 4′1¾″ and 50 lbs, corresponding to the 81st and 50th percentiles when compared to girls her age. She responded well to treatment with various forms of methylphenidate, utilizing at various times both short-acting formulations, as well as a trial on a transdermal patch. A side-effect suffered by the girl was suppression of appetite and a lack of significant weight gain. At three months following the initiation of treatment for ADHD, the girl was 4′2⅞ and 50½ lbs, corresponding to the 85th and 34rd percentiles when compared to girls her age. At one year, she was 4′4⅜″ and 53 lbs, corresponding to the 84th and 36th percentiles when compared to girls her age. At two years she was 4′5¾″ and 54 lbs, corresponding to the 72nd and 17th percentiles when compared to girls her age. At four years of treatment sh...
example 3
Treatment of Patients Suffering from ADHD and Reduction in Appetite
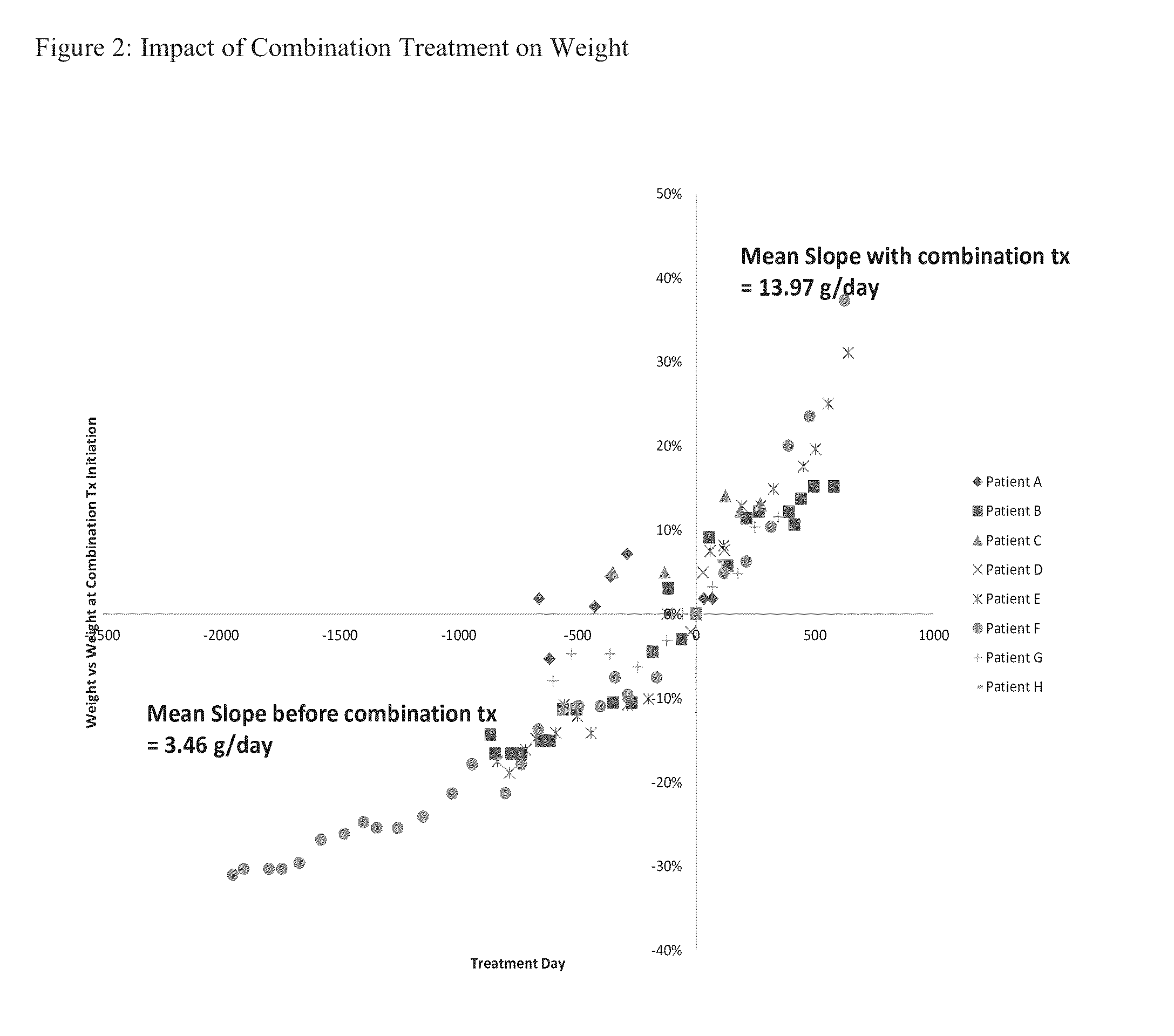
[0167]Eight patients, identified as patients A-H, that came in suffering from ADHD were treated for ADHD. Each patient was prescribed an amphetamine or methylphenidate to treat ADHD and each patient following such treatment suffered a reduction in appetite and failed to gain sufficient weight. Each patient was then prescribed an appetite simulant to be taken along with the amphetamine or methylphenidate. The patients' weight, height and attentiveness were then followed during the course of treatment.
[0168]The age range of the patients was from 6-15 years of age, with a mean age at the start of treatment for ADHD of 8.4 years of age. The mean age at the start of the combination treatment wherein the patient was administered an amphetamine or methylphenidate and an appetite stimulant was 10.3 years of age. The mean treatment period for the eight patients was 1,108 days with a mean of 2.55 ADHD medication changes during...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com