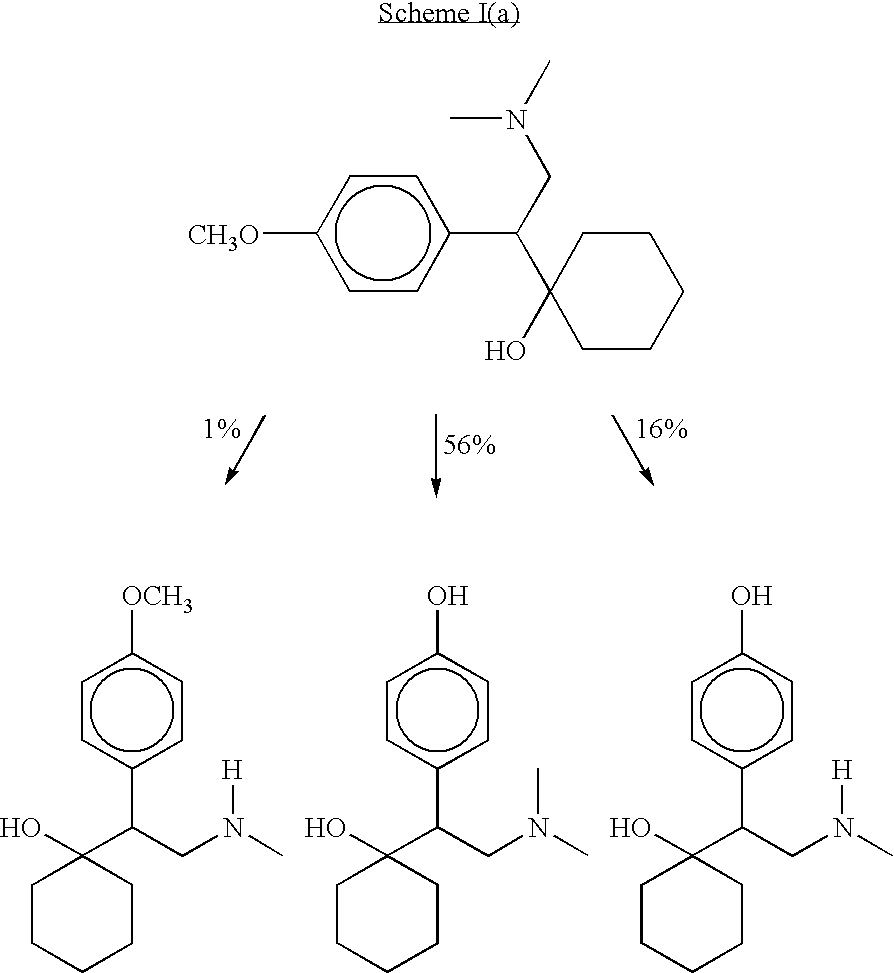
Dosage forms of O-desmethylvenlafaxine
a technology of o-desmethylvenlafaxine and derivatives, which is applied in the field of derivatives of racemic venlafaxine, can solve the problems of affecting the study of the activity of o-desmethylvenlafaxine as compared to its parent, affecting the effect of drug administration, so as to achieve the effect of reducing or avoiding adverse effects, potent activity, and avoiding adverse effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
5.1. Example 1
Synthesis of Venlafaxine
1-[cyano-4-methoxyphenyl)methyl]cyclohexanol
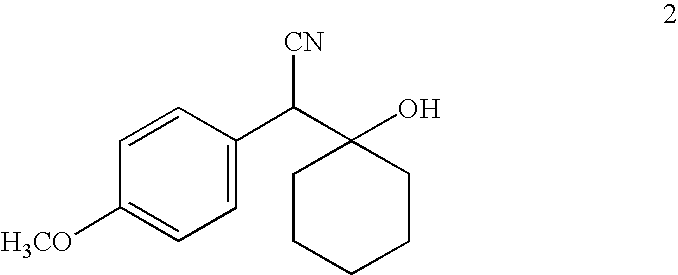
[0075]A solution of 4-methoxybenzylnitrile (53.5 g, 0.36 mol) in 400 mL THF was cooled to −78° C. followed by slow addition of a 2.0 M THF solution of lithium diisopropylamide (200 mL, 0.40 mol) maintaining the reaction temperature below −65° C. The reaction was stirred at −78° C. for 30 minutes. Cyclohexanone (39.5 g, 0.40 mol) was added at a rate such that the reaction temperature did not rise above −65° C. After the addition reaction was stirred at −78° C. for 2 hours, then was poured into 1 L saturated aqueous NH4Cl containing ice. The mixture was stirred for 15 minutes and was extracted with ethyl acetate (4×200 mL). Combined ethyl acetate layer was washed with water (3×100 mL), brine (1×100 mL) and dried (Na2SO4). Ethyl acetate was evaporated in vacuo to give colorless solid that was triturated with hexane. The precipitate was filtered, washed with hexane, dried in vacuo to give colorless solid (...
example 2
5.2. Example 2
Synthesis of (±)-O-desmethylvenlafaxine
[0079]A solution of diphenylphosphine (3.0 g, 16.1 mmol) in 20 mL THF was cooled to −10° C. followed by slow addition of a 1.6 M THF solution of n-BuLi (12.7 mL, 20.2 mmol) at a rate such that reaction temperature did not rise above 0° C. The reaction was stirred at 0° C. for 30 minutes. A solution of (±)-venlafaxine (1.0 g, 3.6 mmol) in 10 mL THF was added slowly at 0° C. The reaction was stirred at 0° C. for 15 minutes and allowed to warm to room temperature and stirred for 1 hour. It was then refluxed overnight. The reaction was cooled to room temperature and was poured slowly into 30 mL cold 3N HCl maintaining the temperature below 15° C. After stirring for 10 minutes, the aqueous layer was extracted with ethyl acetate (3×30 mL). The aqueous layer was adjusted to pH 6.8-6.9 by slow addition of solid NaHCO3. It was then saturated by adding NaCl and was extracted with ethyl acetate (6×30 mL). Combined ethyl acetate layer was dri...
example 3
5.3. Example 3
Synthesis of (±)-N-desmethylvenlafaxine
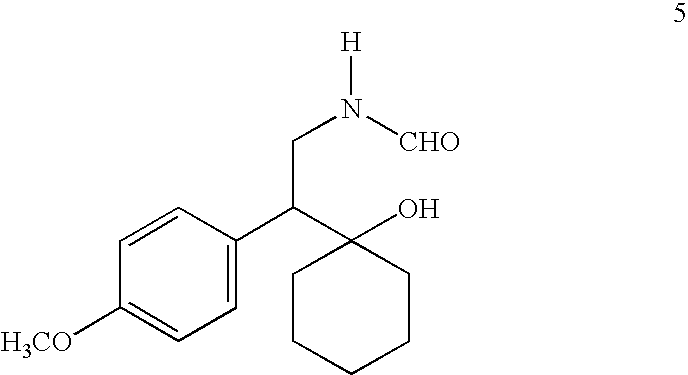
[0080]To a solution of 1-[amino(4-methoxyphenyl)ethyl]cyclohexanol (1.0 g, 4.0 mmol) in 8 mL of toluene, 96% formic acid (0.37 g, 8.0 mmol) was added and the reaction was refluxed for 4 hours. It was cooled to room temperature and poured into 40 mL saturated aqueous NaHCO3. Toluene layer was separated and aqueous layer was extracted with toluene (3×15 mL). Combined toluene layer was washed with water (3×15 mL), brine (1×15 mL) and dried (Na2SO4). Toluene was evaporated in vacuo to give crude N-formyl compound as yellow gum (0.930 g, 83.8% yield). 1H (CDCl3): 7.95 (s, 1H), 7.15 and 6.85 (q, 4H), 5.80 (s, 1H), 4.10 (m, 1H), 3.80 (s, 3H), 3.50 (s, 1H), 2.80 (dd, 1H), 1.50 (m, 10H); 13C (CDCl3): 161.4, 158.8, 131.0, 130.7, 113.9, 73.0, 55.3, 54.2, 38.1, 36.1, 35.6, 25.6, 21.9, 21.8. (Impurity: 164.5, 129.0, 128.0, 125.0, 56.5, 42.0, 36.5, 35.5). MS (277, M+).
[0081]To a solution of crude N-formyl compound (0.585 g, 2.1 mmol) in 6 mL TH...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight percent | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com