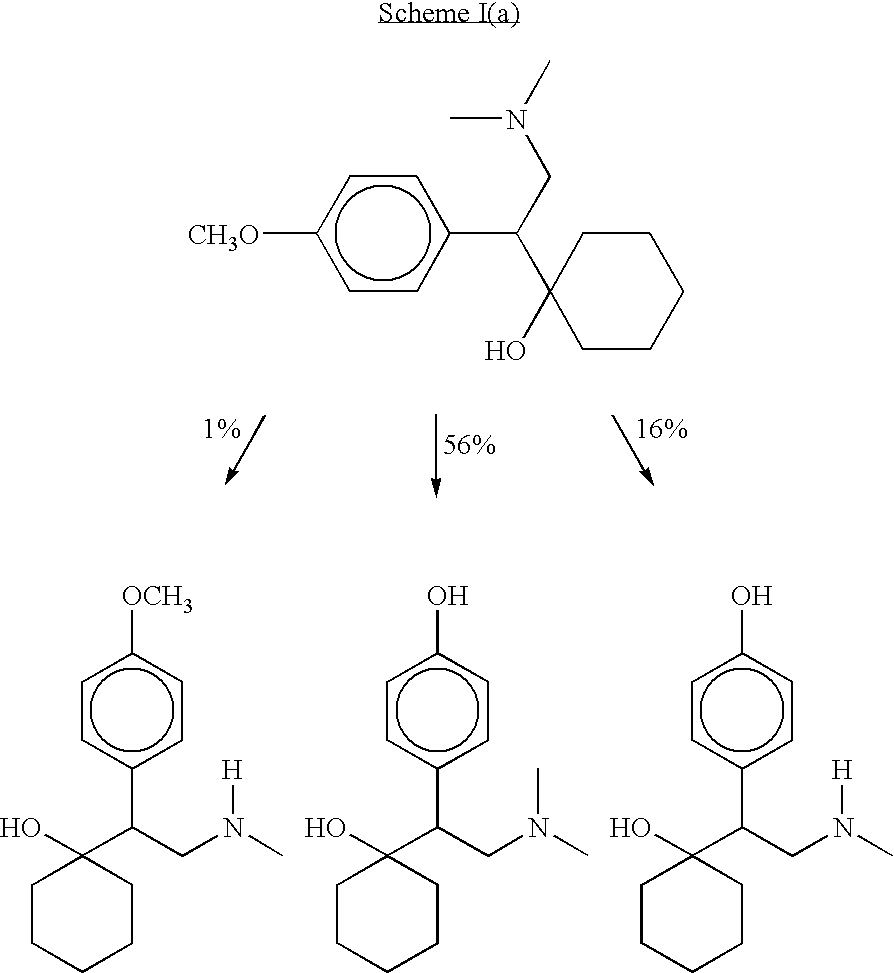
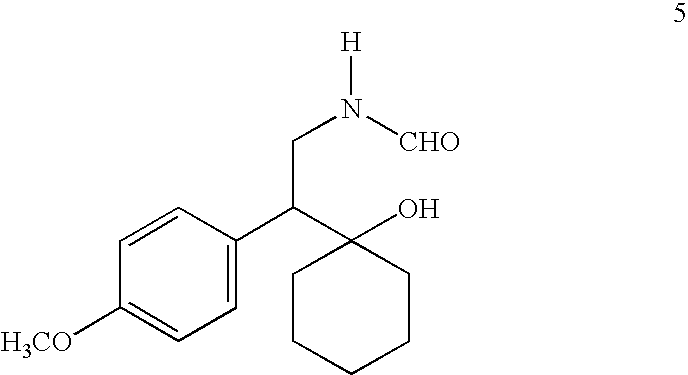
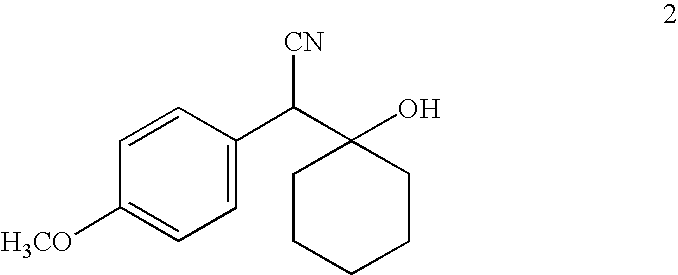
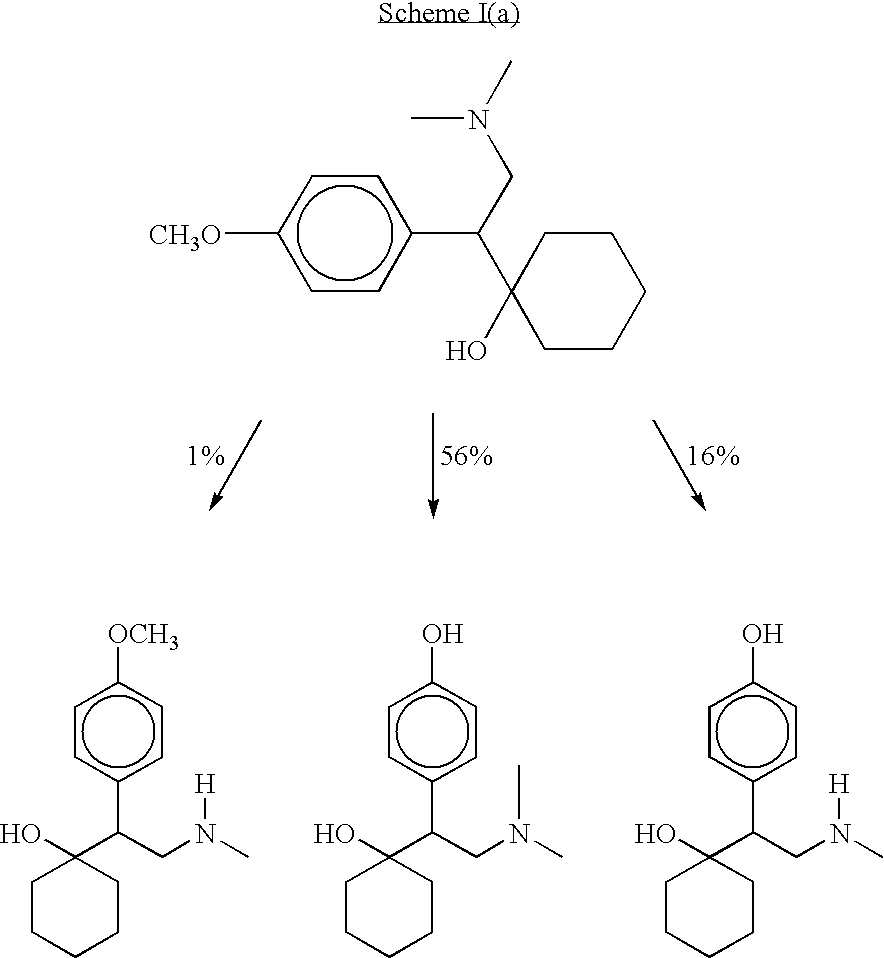
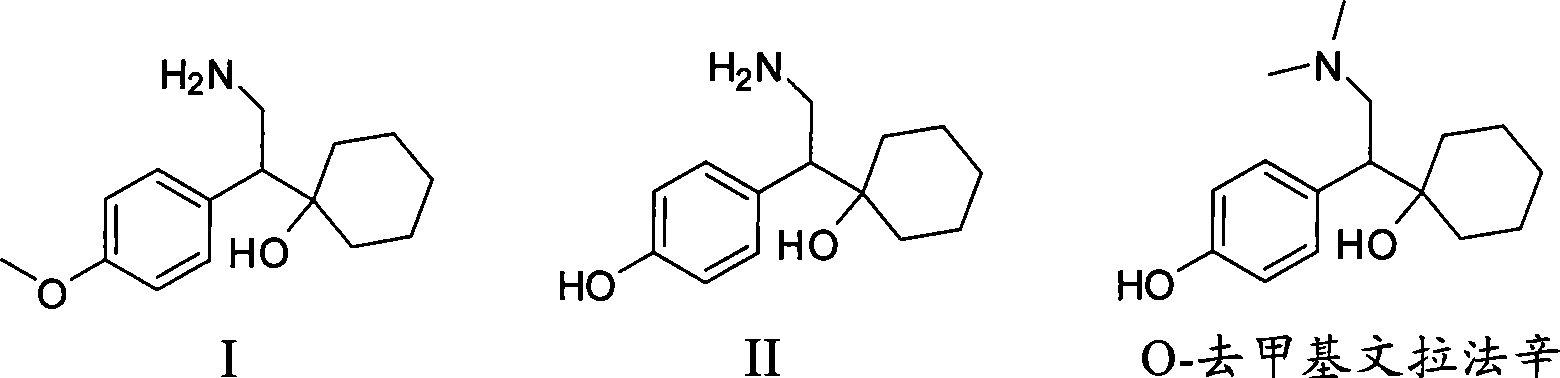
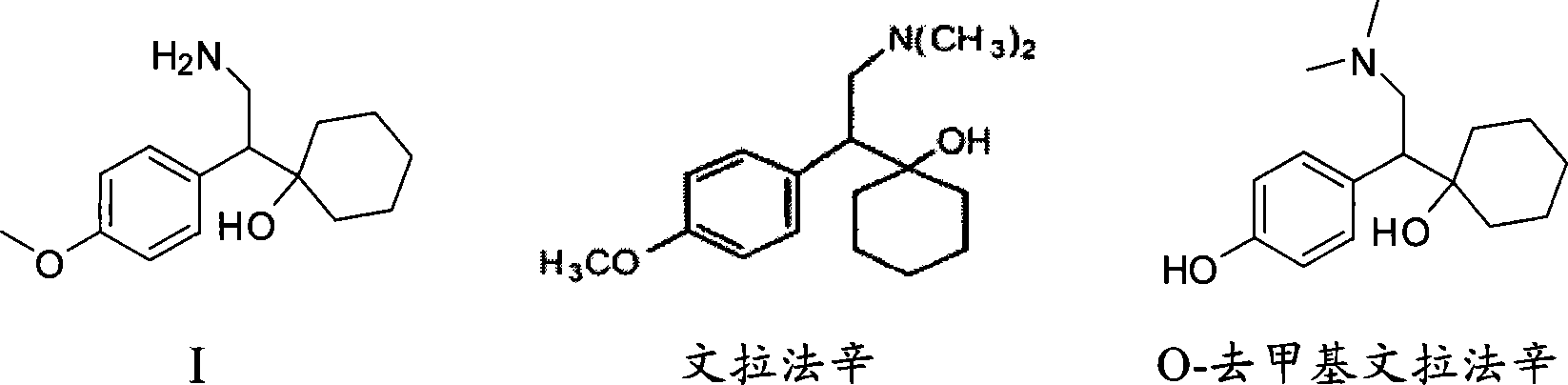
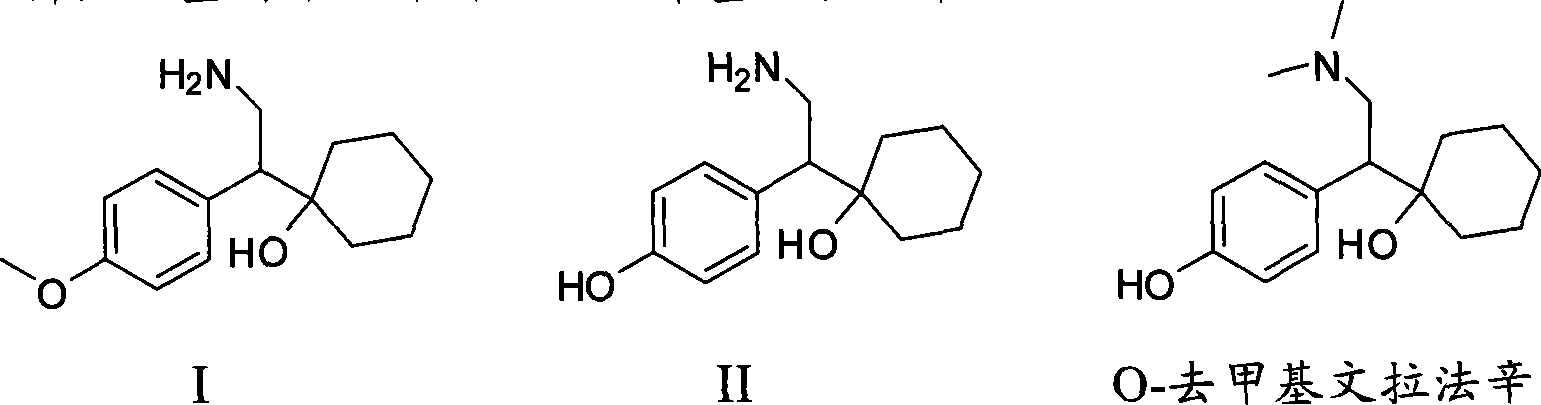
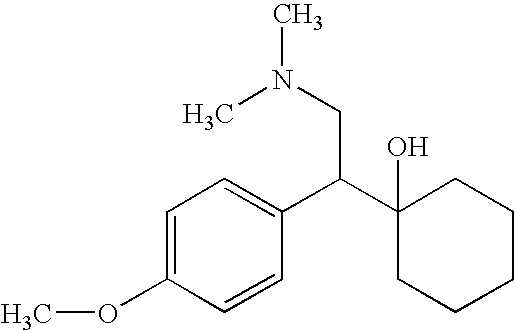
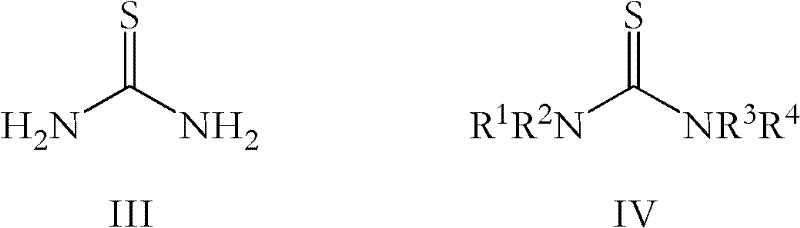Patents
Literature
72 results about "O desmethylvenlafaxine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
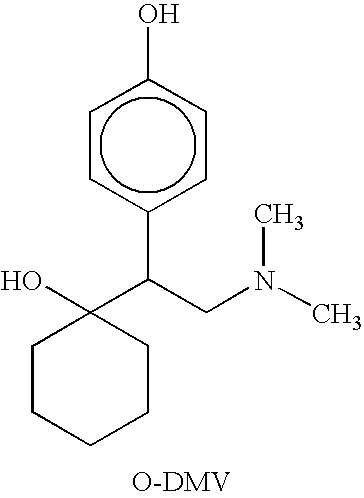
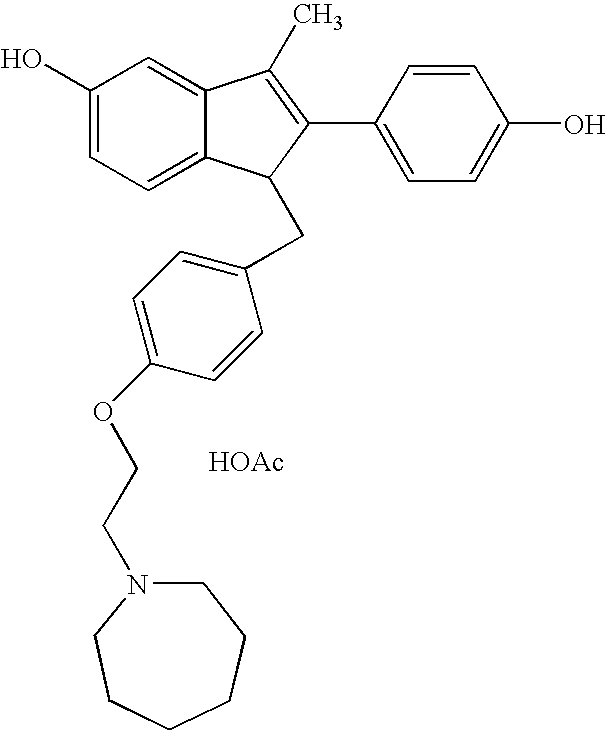
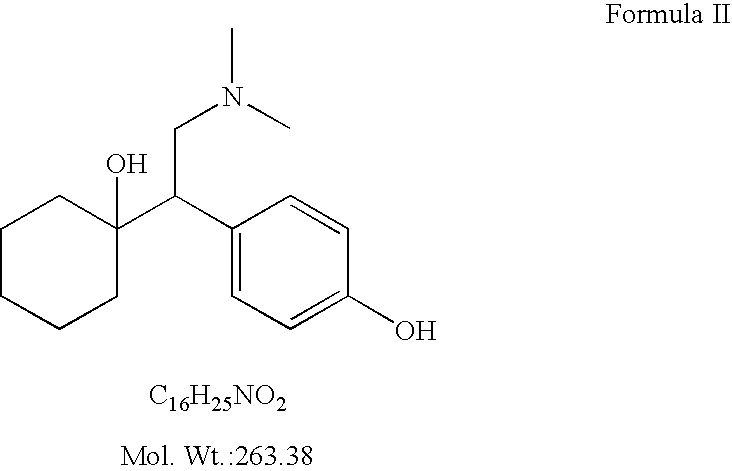
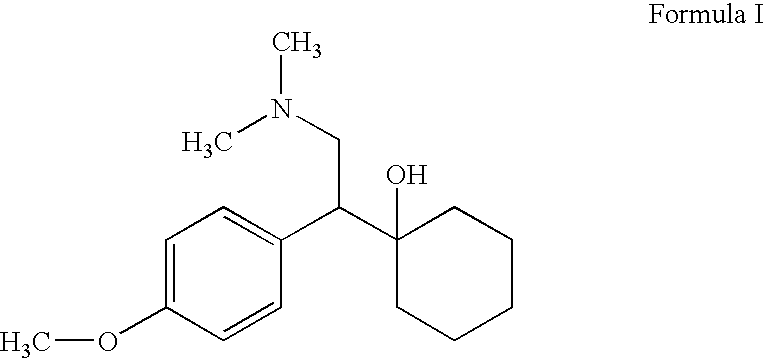
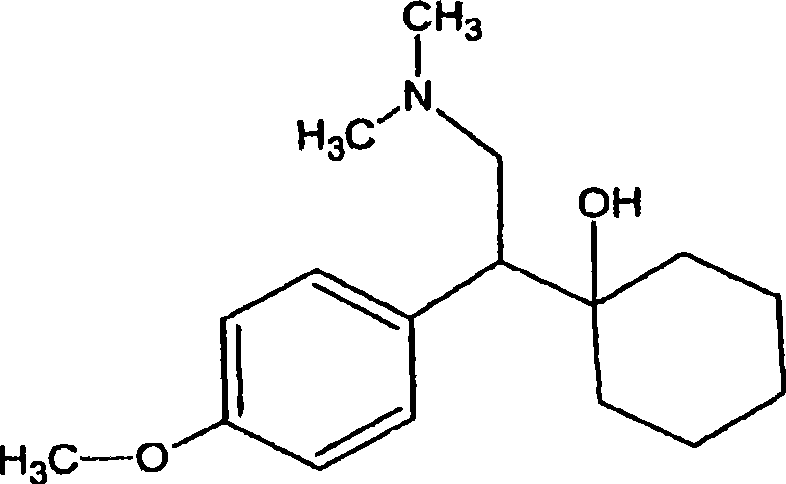
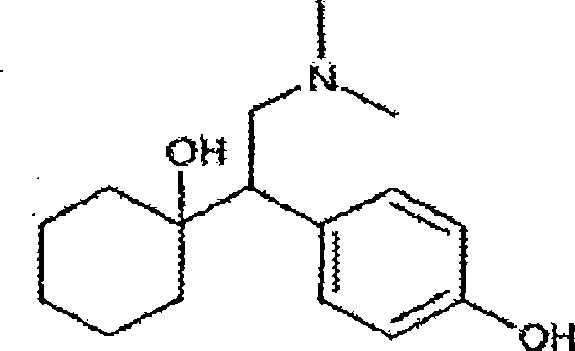
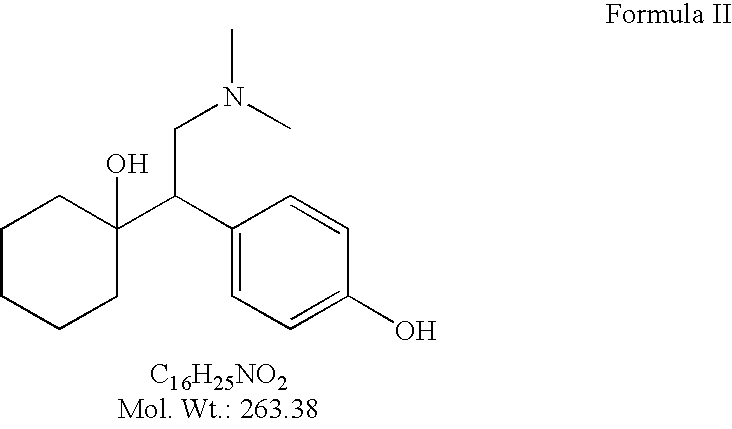
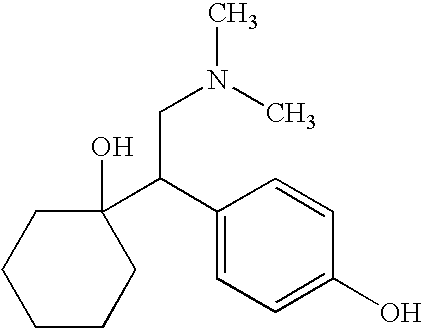
O-desmethylvenlafaxine: AHFS/Drugs.com: ... Desvenlafaxine, sold under the brand name Pristiq among others, ... When most normal metabolizers take venlafaxine, approximately 70% of the dose is metabolized into desvenlafaxine, so the effects of the two drugs are expected to be very similar.
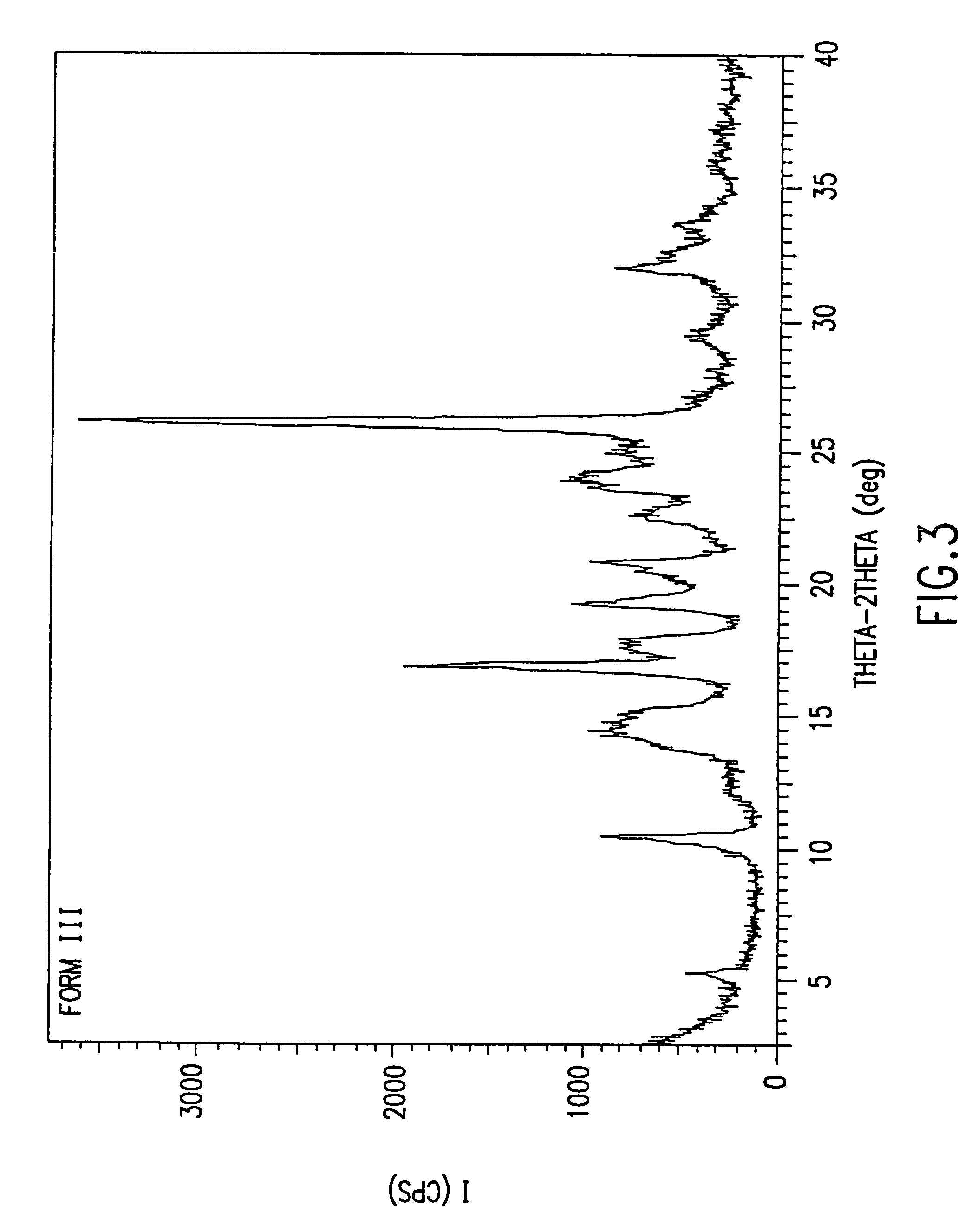
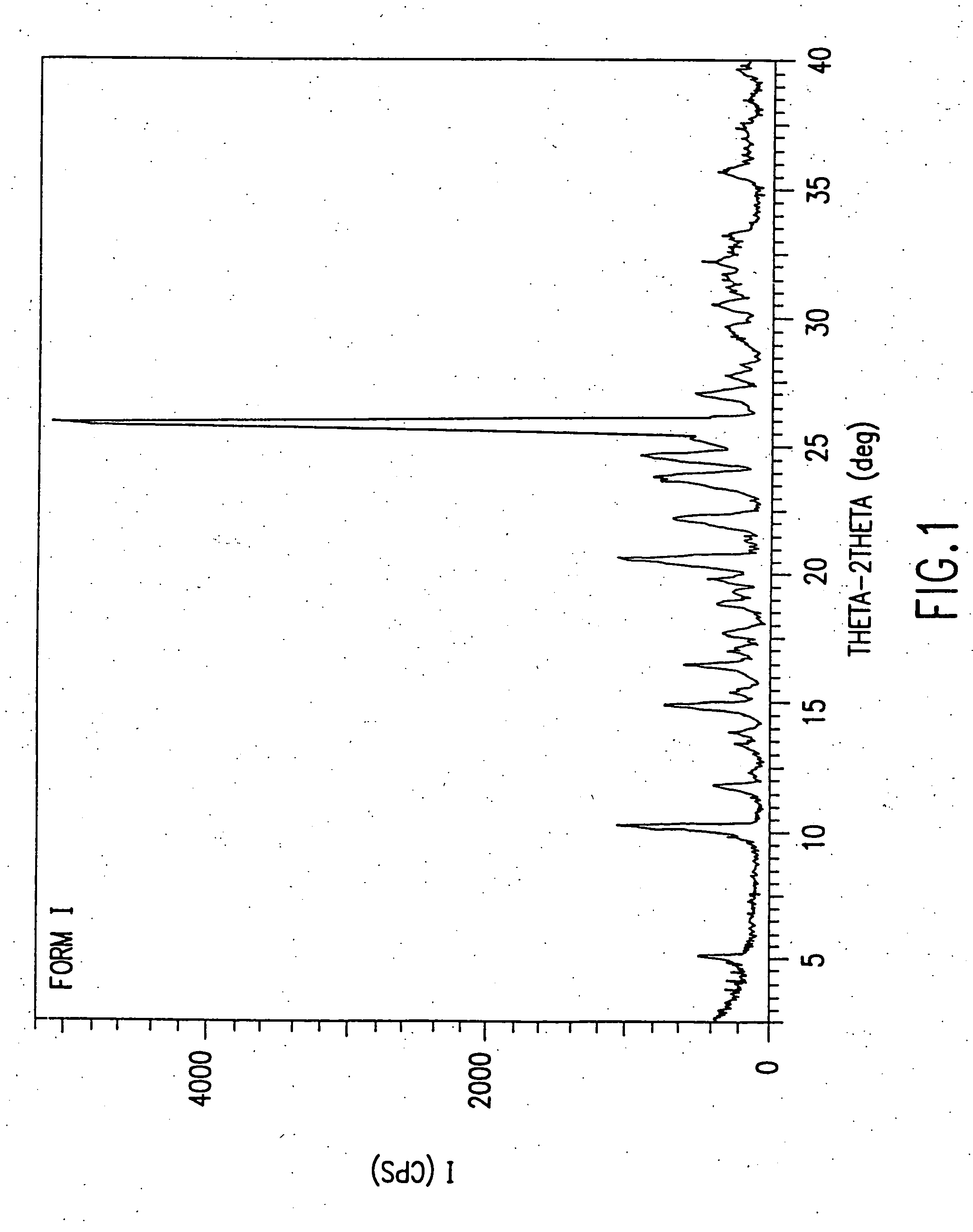
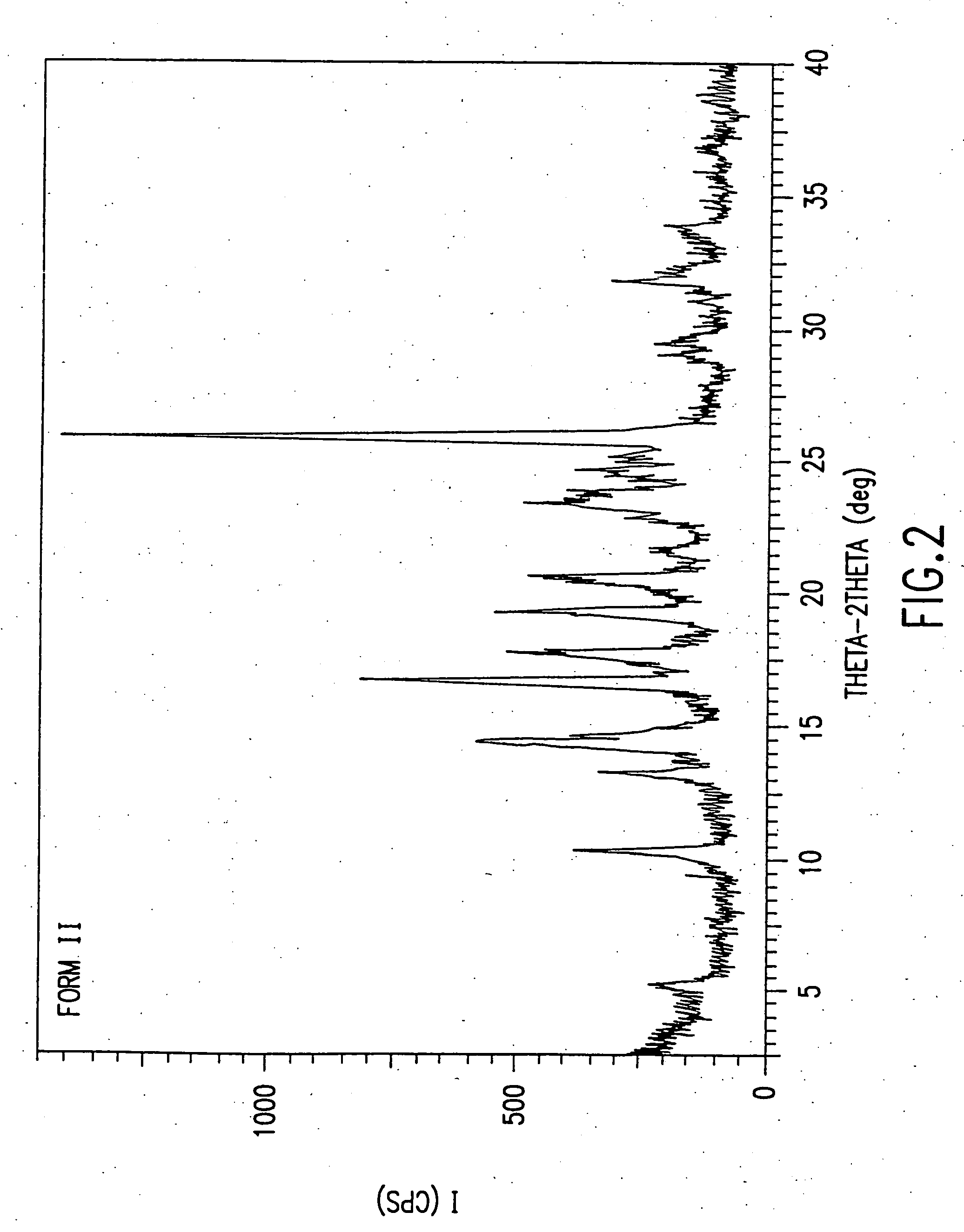
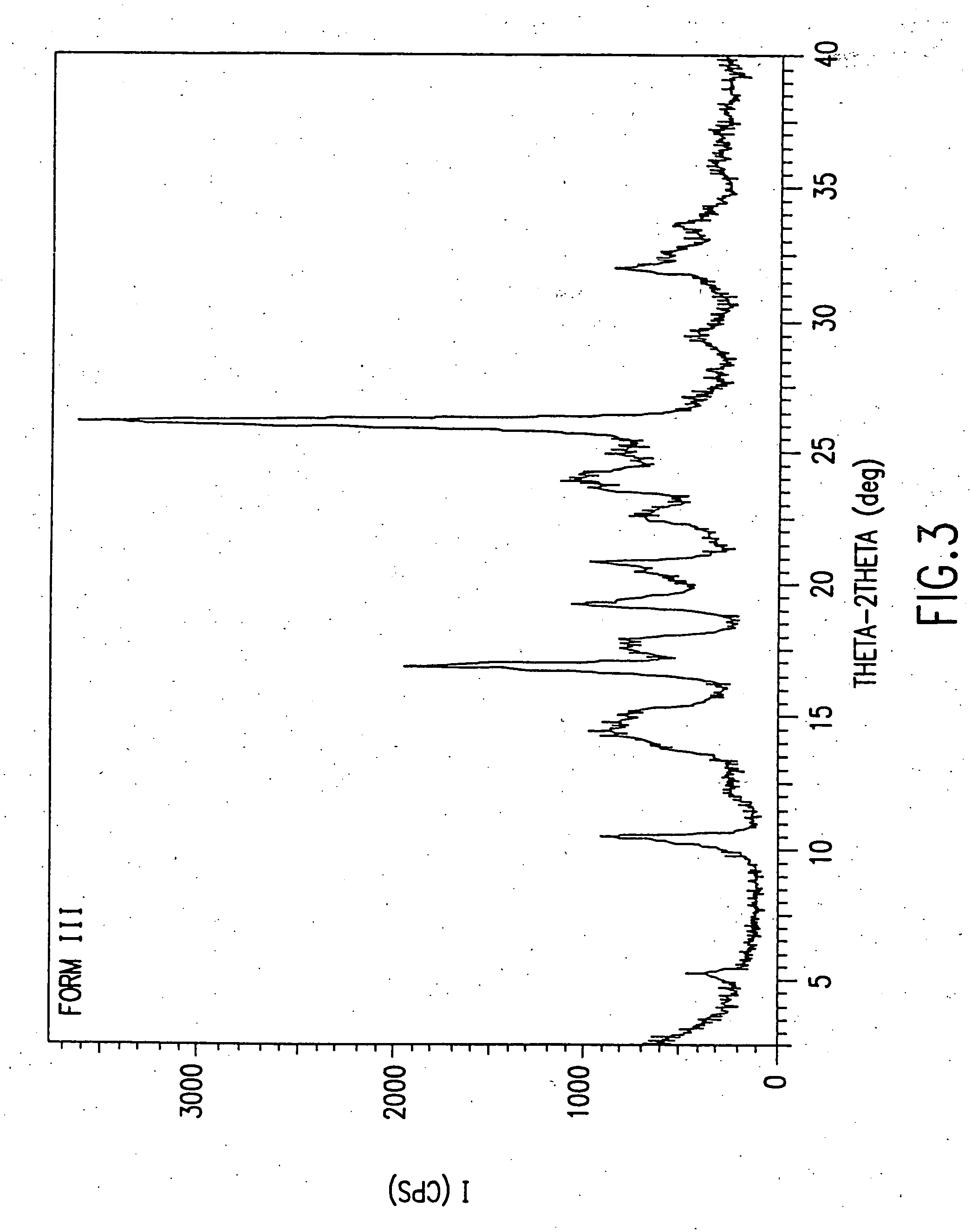
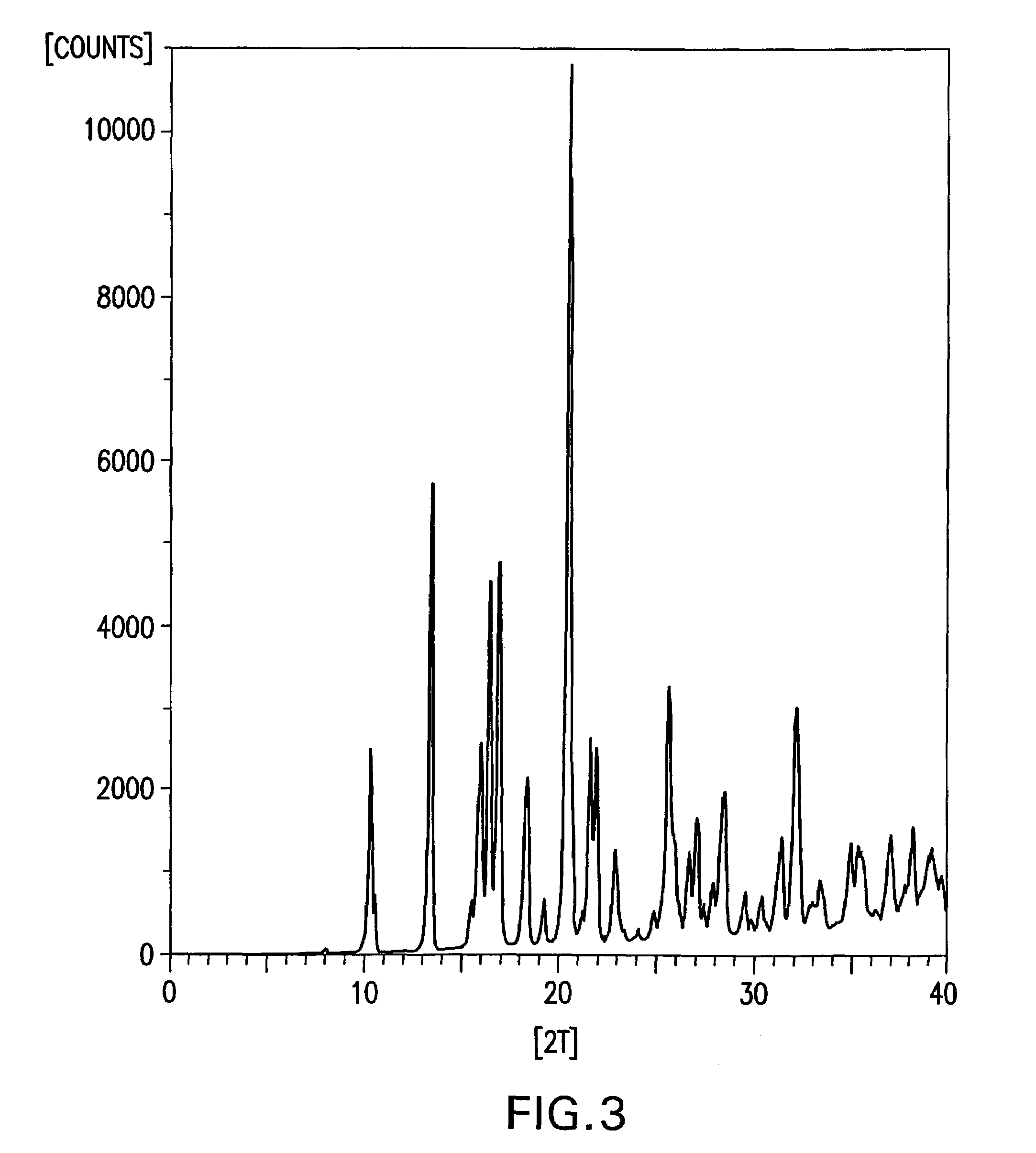
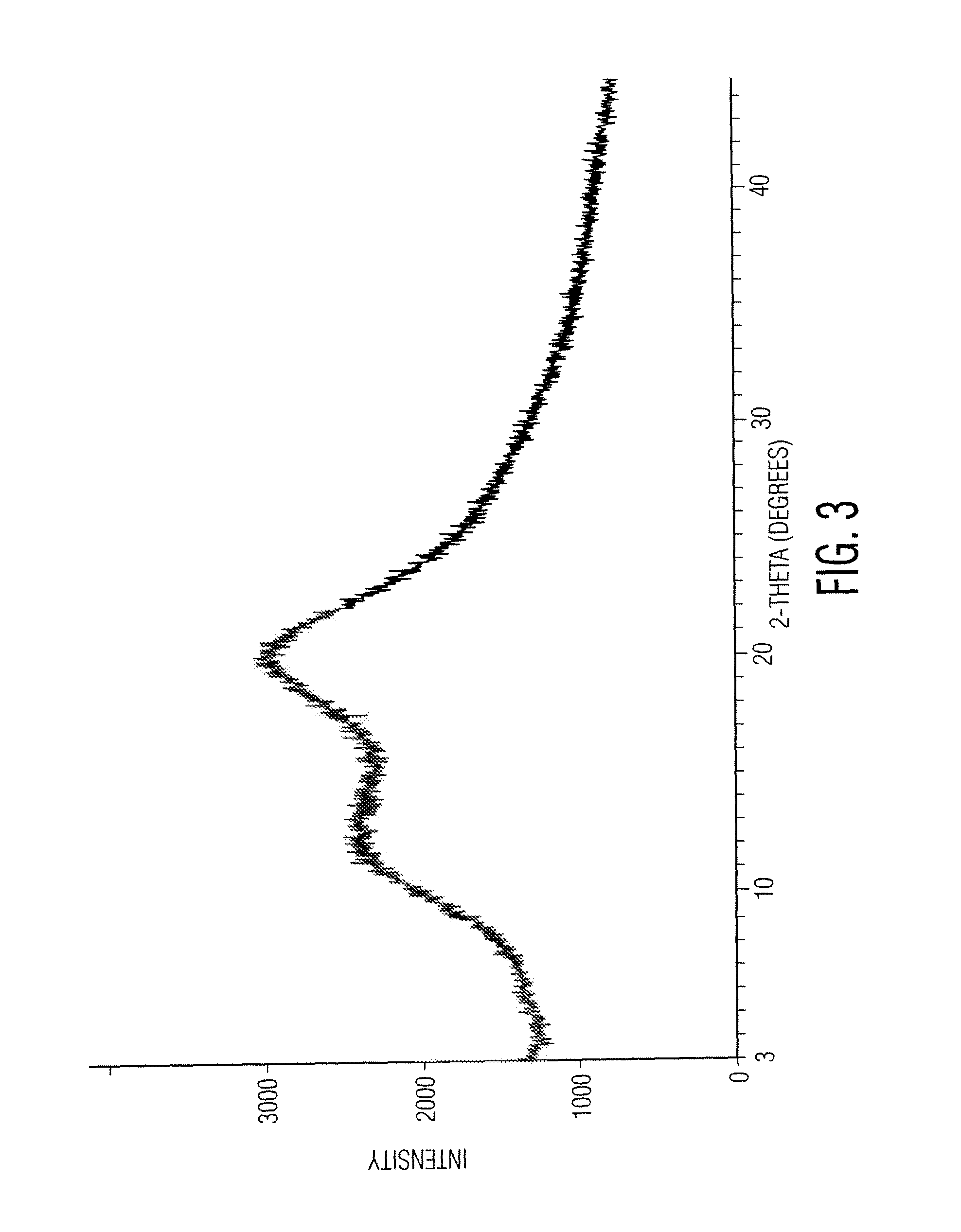
Dosage forms of O-desmethylvenlafaxine
InactiveUS20080132578A1Potent activityReducing and avoiding adverse effectOrganic active ingredientsBiocideClinical psychologyAttention deficits
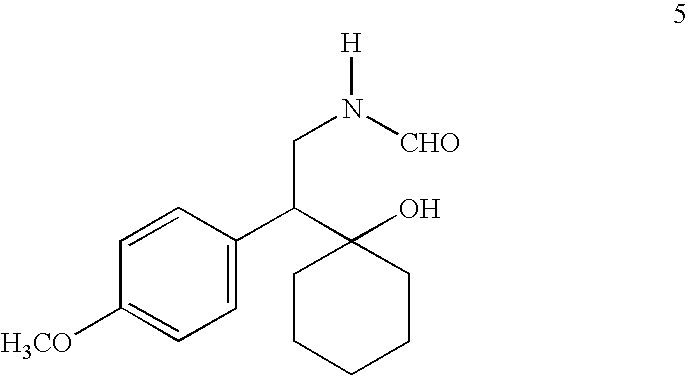
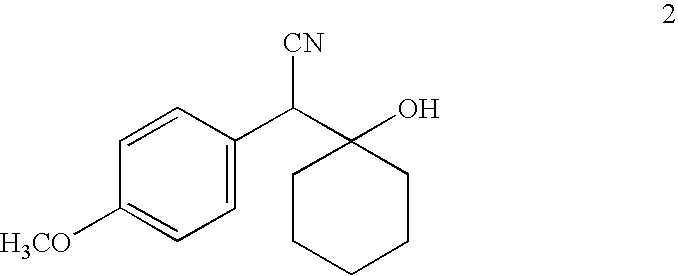
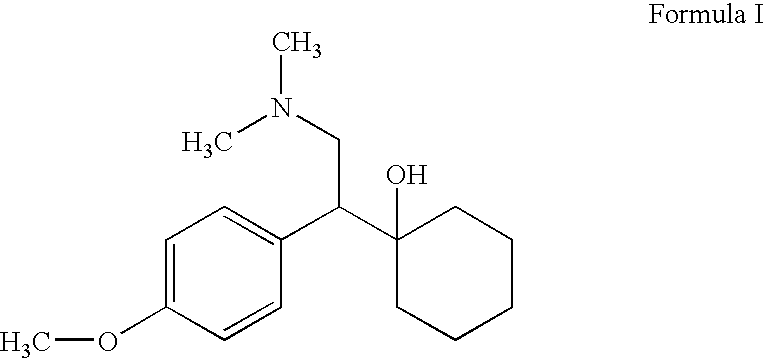
Methods of preparing, and compositions comprising, derivatives of venlafaxine are disclosed. Also disclosed are methods of treating and preventing diseases and disorders including, but not limited to, affective disorders such as depression, bipolar and manic disorders, attention deficit disorder, attention deficit disorder with hyperactivity, Parkinson's disease, epilepsy, cerebral function disorders, obesity and weight gain, incontinence, dementia and related disorders.
Owner:WYETH LLC
O-desmethylvenlafaxine and methods of preparing and using the same
InactiveUS20050197392A1Reduce weightPotent activityBiocideNervous disorderAttention deficitsEpileptic disorder
Methods of preparing, and compositions comprising, derivatives of venlafaxine are disclosed. Also disclosed are methods of treating and preventing diseases and disorders including, but not limited to, affective disorders such as depression, bipolar and manic disorders, attention deficit disorder, attention deficit disorder with hyperactivity, Parkinson's disease, epilepsy, cerebral function disorders, obesity and weight gain, incontinence, dementia and related disorders.
Owner:WYETH LLC
Controlled release O-desmethylvenlafaxine formulations
InactiveUS20060193912A1Lower Level RequirementsLower potentialOrganic active ingredientsPill deliveryControlled releaseDosage form
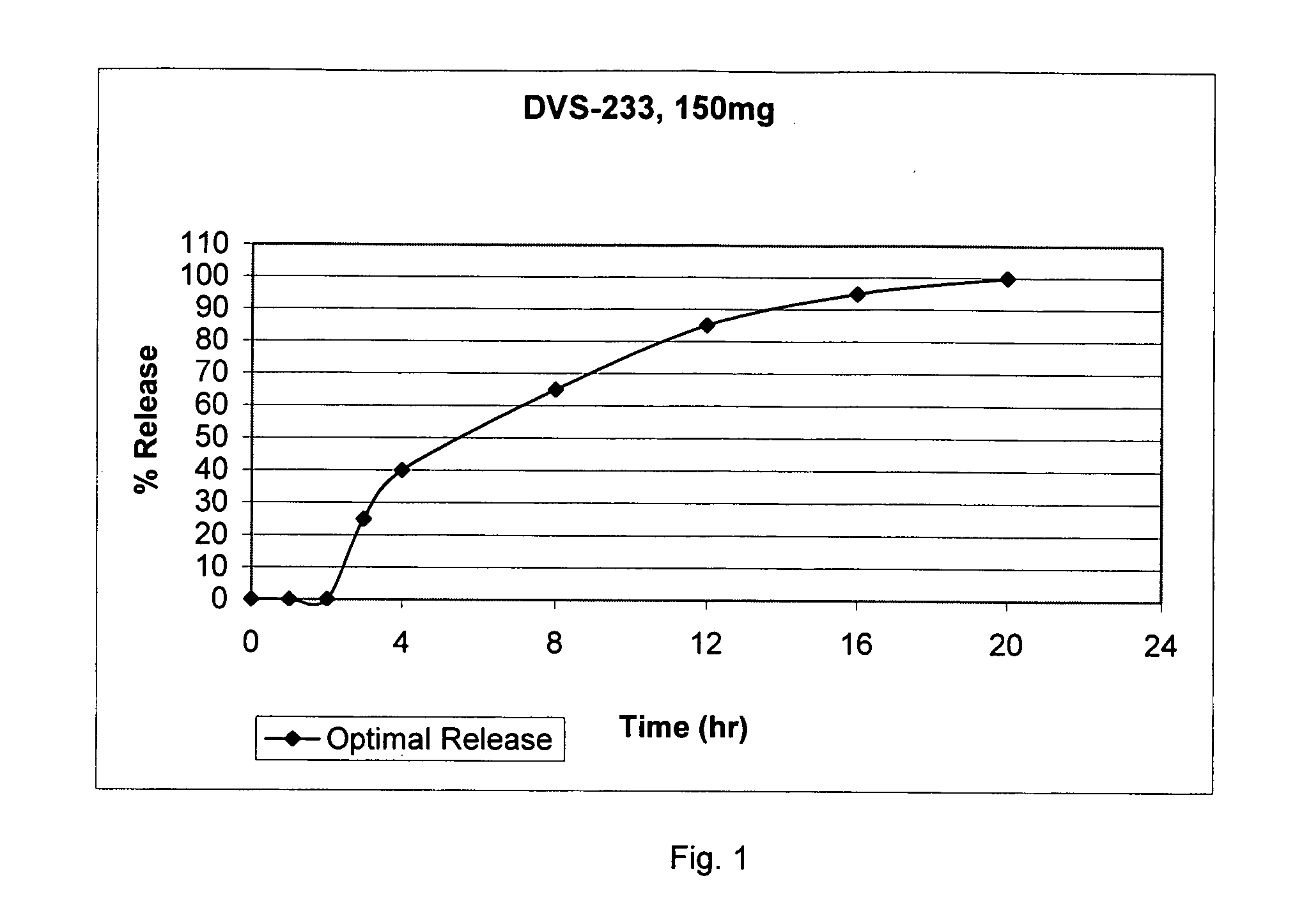
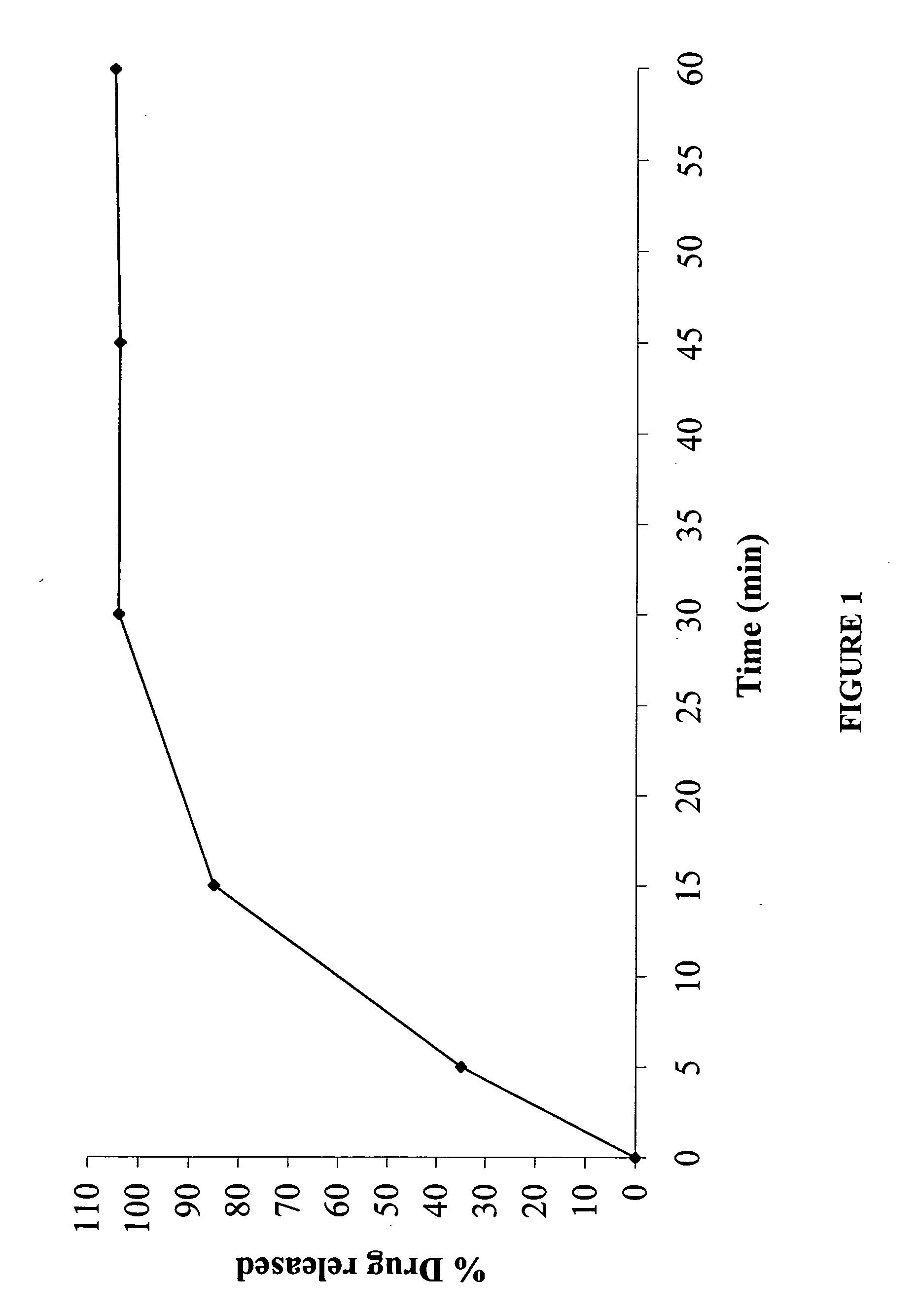
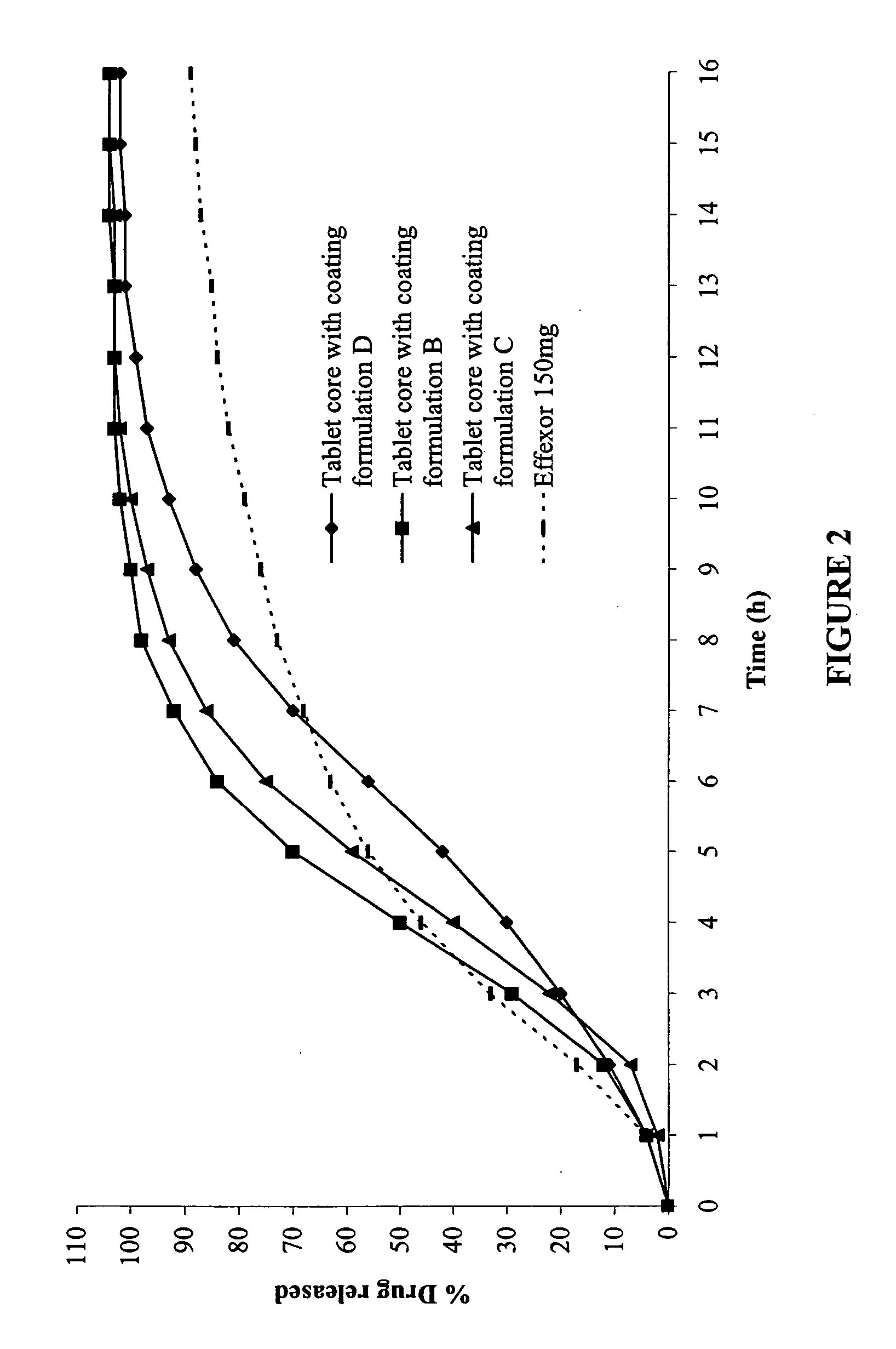
In certain embodiments, the present invention is directed to a controlled release oral dosage form comprising a therapeutically effective amount of O-desmethylvenlafaxine or a pharmaceutically acceptable salt thereof and a controlled release material; wherein the amount of O-desmethylvenlafaxine or pharmaceutically acceptable salt thereof released at 1 hour in 900 mL 0.1 N HCl (pH 1.5) with 40% EtOH using USP Apparatus II at 50 rpm is within 25% of the amount of O-desmethylvenlafaxine or pharmaceutically acceptable salt thereof released at 1 hour in 900 mL 0.1 N HCl (pH 1.5) using USP Apparatus II at 50 rpm.
Owner:PENWEST PHARMA CO
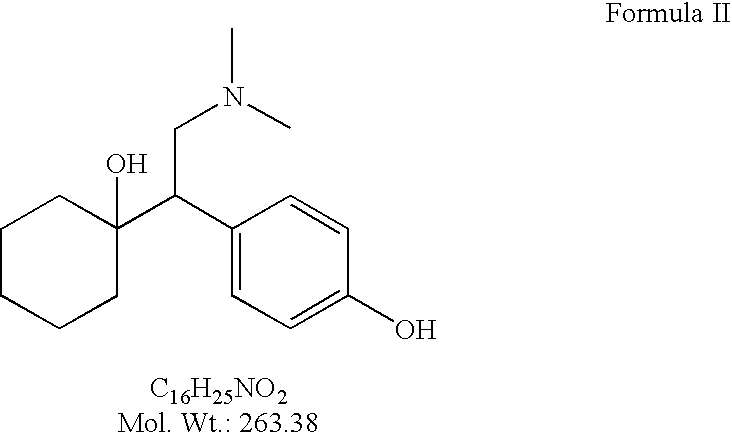
Succinate salt of O-desmethyl-venlafaxine
InactiveUS7026508B2Suitable for useImprove bioavailabilityOrganic active ingredientsNervous disorderSuccinatesDesmethyl
A novel salt of O-desmethyl venlafaxine is provided, O-desmethylvenlafaxine succinate. Pharmaceutical compositions, dosage forms and methods of use are also provided.
Owner:WYETH LLC
Multiparticulate O-desmethylvenlafaxine salts and uses thereof
InactiveUS20050175698A1Undesirable characteristicDosing is convenientBiocideNervous disorderSide effectFormate
A multiparticulate O-desmethylvenlafaxine (ODV) succinate or formate is described. Methods of treating depression and reducing the gastrointestinal side-effects of ODV are also described.
Owner:WYETH LLC
Extended release pharmaceutical dosage form
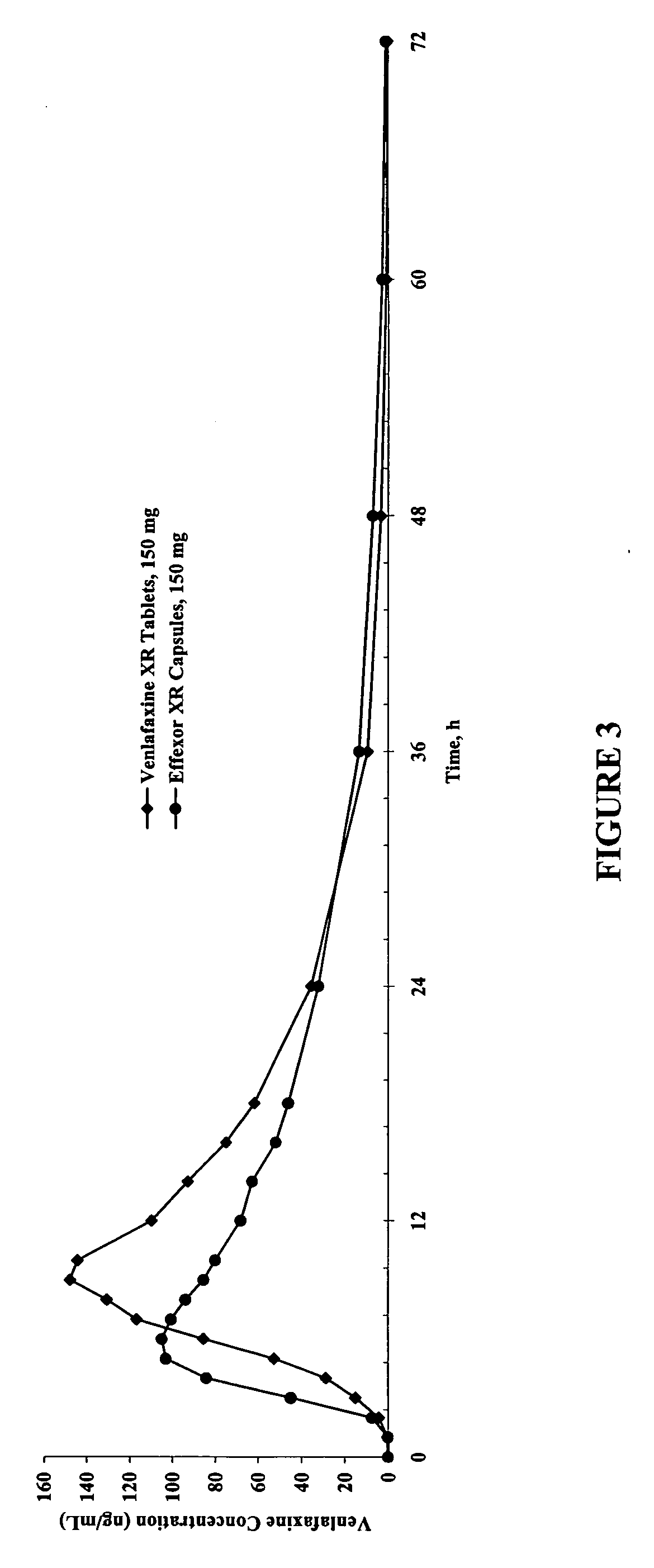
This invention relates to novel extended release pharmaceutical dosage forms for orally delivering drugs to mammals, e.g., humans. More particularly, this invention concerns novel dosage forms of water soluble drugs such as venlafaxine, enantiomeric (R or S) forms of venlafaxine, metabolites of venlafaxine such as O-desmethyl venlafaxine (ODV) or enantiomeric (R or S) forms of said metabolites which dosage forms have an extended release profile when taken orally. This invention also provides processes for preparing such dosage forms and methods of using them.
Owner:WYETH LLC
Highly bioavailable oral delayed release dosage forms of O-desmethylvenlafaxine succinate
InactiveUS20070014859A1Improve bioavailabilityUndesirable side-effectOrganic active ingredientsNervous disorderDelayed Release Dosage FormSide effect
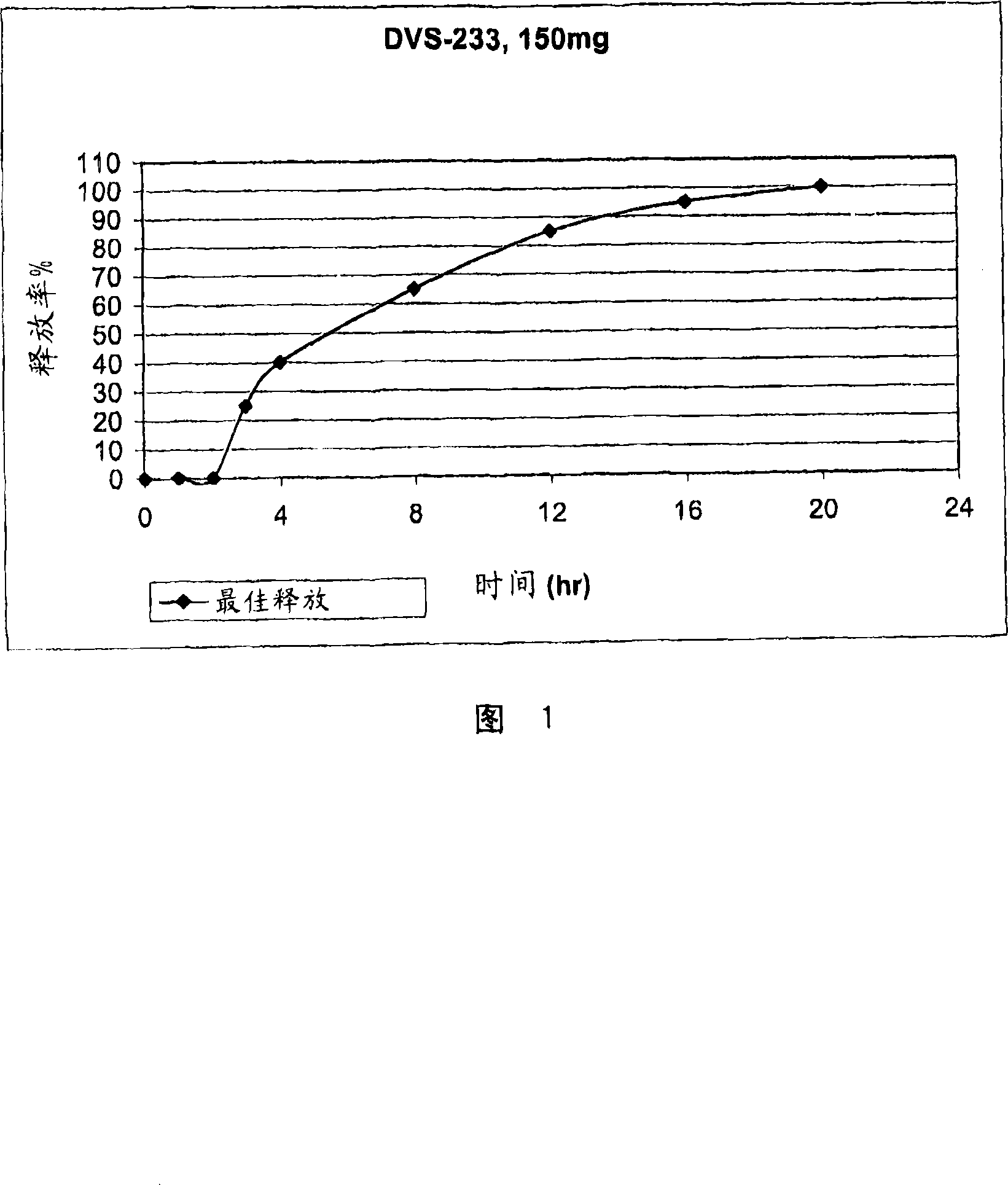
An oral, highly bioavailable unit dosage form of O-desmethylvenlafaxine succinate (DVS) having a delayed release of at least about one hour and a sustained release over multiple hours to provide a total release of greater than about 85% within about 12 to about 14 hours is described. In one embodiment, the superbioavailable DVS composition has a delayed release of about two hours and a total release of greater than about 95% within about 12 to about 14 hours. Use of the formulation in treating depression and reducing the gastrointestinal side-effects of O-desmethylvenlafaxine (ODV) is also described.
Owner:WYETH LLC
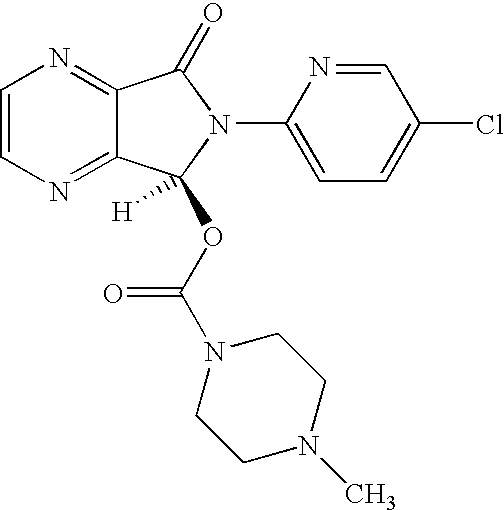
Combinations of Eszopiclone and O-Desmethylvenlafaxine, and Methods of Treatment of Menopause and Mood, Anxiety, and Cognitive Disorders
One aspect of the present invention relates to pharmaceutical compositions containing two or more active agents that when taken together can be used to treat, e.g., menopause, mood disorders, anxiety disorders, or cognitive disorders. The first component of the pharmaceutical composition is a sedative eszopiclone. The second component of the pharmaceutical composition is O-desmethylvenlafaxine. The present invention also relates to a method of treating menopause, perimenopause, mood disorders, anxiety disorders, and cognitive disorders.
Owner:SEPACOR INC
Novel succinate salt of O-desmethyl-venlafaxine
InactiveUS20050096479A1Improve solubilityImprove permeabilityOrganic active ingredientsNervous disorderSuccinatesDesmethyl
A novel salt of O-desmethyl venlafaxine is provided, O-desmethylvenlafaxine succinate. Pharmaceutical compositions, dosage forms and methods of use are also provided.
Owner:WYETH
Modified-release compositions of at least one form of venlafaxine
InactiveUS20050244498A1Reduce morbidityReduce releaseBiocideAnimal repellantsControlled releaseOral medication
The present invention relates to a modified release composition of at least one form of venlafaxine, which is an enhanced absorption delayed controlled release composition for oral administration suitable for once daily dosing. The composition comprises a core comprising at least one form of venlafaxine selected from the group consisting of venlafaxine, an active metabolite of venlafaxine, a pharmaceutically acceptable salt of venlafaxine, a pharmaceutically acceptable salt of an active metabolite of venlafaxine, and combinations thereof, and a pharmaceutically acceptable excipient. The composition further comprises a modified release coating which substantially surrounds the core. The compositions of the invention provide enhanced absorption delayed controlled release of the at least one form of venlafaxine such that the combined geometric mean ratio of the composition of the invention to the reference product for the AUC0-t or the Cmax for venlafaxine and its active metabolite O-desmethylvenlafaxine is greater than 2 after first administration of the composition under fed or fasting conditions.
Owner:BIOVAIL LAB INT SRL
Formate salt of O-desmethyl-venlafaxine
A novel salt of O-desmethylvenlafaxine, O-desmethylvenlafaxine formate, is provided. Pharmaceutical compositions, dosage forms and methods of use are also provided.
Owner:WYETH LLC
Transdermal drug delivery devices containing O-Desmethyl Venlafaxine (ODV) or its salts
InactiveUS20070053968A1Simple and convenient and non-invasive administrationFast deliveryOrganic active ingredientsBiocideVasomotor symptomSerotonin
The present invention provides transdermal drug delivery devices (i.e., patches) comprising O-desmethylvenlafaxine (ODV), a selective serotonin and norepinephrine re-uptake inhibitor, or a pharmaceutically acceptable salt thereof, which, among other, offer the advantage of eliminating or reducing the adverse side effects associated with the oral administration of ODV. Also provided are methods of preparing and using these transdermal delivery systems for the treatment of depression, anxiety disorders, vasomotor symptoms and pain.
Owner:WYETH LLC
Kit for detecting anti-depressant drugs in serum and plasma by liquid chromatography tandem mass spectrometry and application thereof
InactiveCN109085264APharmacologically activeInterpret blood levelsComponent separationSertralineTandem mass spectrometry
The invention provides a kit for detecting anti-depressant drugs in serum and plasma by liquid chromatography tandem mass spectrometry. The kit comprises drug standard substances, drug internal standardization compounds, drug extraction compositions, negative plasma and a diluent. The drug standard substances comprise amfebutamone, oxybupropion, citalopram, Escitalopram, venlafaxine, O-desmethylvenlafaxine, duloxetine, fluoxetine, norfloxetine, fluvoxamine, mirtazapine, paroxetine, sertraline and trazodone. The drug internal standardization compounds comprise amfebutamone-d9, oxybupropion-d6,citalopram-d6, venlafaxine-d6, O-desmethylvenlafaxine-d6, duloxetine-d3, fluoxetine-d6, norfloxetine-d6, fluvoxamine-d4, mirtazapine-d3, paroxetine-d6, sertraline-d3 and trazodone-d6. The drug extraction compositions comprise, by volume, 60% of methanol solution, 20% of acetonitrile solution, 10% of isopropyl alcohol solution and 10% of purified water. The diluent comprises 50 % of methanol waterfluid. The kit can be used for simultaneous detection of the anti-depressant drugs and active metabolites, the detection time is short, and flux is high.
Owner:HANGZHOU BAICHEN MEDICAL INSTR CO LTD +1
Highly bioavailable oral delayed release dosage forms of O-desmethylvenlafaxine succinate
InactiveCN101247791AOrganic active ingredientsNervous disorderDelayed Release Dosage FormSide effect
Owner:WYETH LLC
O-desmethylvenlafaxine and bazedoxifene combination product and uses thereof
A combination product containing at least two active compounds, O-desmethylvenlafaxine or a pharmaceutically acceptable salt thereof and bazedoxifene or a pharmaceutically acceptable salt thereof is described. Also described are methods of making and using this combination product to treat a variety of conditions associated with low circulating estrogen levels or low estrogen receptor activity.
Owner:WYETH LLC
Processes for the synthesis of O-desmethylvenlafaxine
InactiveUS20090069601A1Organic compound preparationAmino compound preparationMedicinal chemistryO desmethylvenlafaxine
Owner:TEVA PHARM USA INC
Novel formate salt of O-desmethyl-venlafaxine
A novel salt of O-desmethylvenlafaxine, O-desmethylvenlafaxine formate, is provided. Pharmaceutical compositions, dosage forms and methods of use are also provided.
Owner:WYETH LLC
Topical formulations containing O-Desmethyl Venlafaxine (ODV) or its salts
InactiveUS20070054964A1Simple and convenient and non-invasive administrationAvoidingBiocideOrganic active ingredientsSerotoninVasomotor symptom
The present invention provides topical compositions comprising O-desmethylvenlafaxine (ODV), a selective serotonin and norepinephrine re-uptake inhibitor, or a pharmaceutically acceptable salt thereof. In certain embodiments, the inventive topical formulations contain one or more percutaneous / permucosal absorption enhancers. Also provided are methods of preparing and using these compositions for the treatment of diseases or conditions where a localized therapeutic effect is sought, such as vasomotor symptoms and pain.
Owner:WYETH LLC
Simple and efficient method for detecting concentration of venlafaxine and active metabolite O-desmethylvenlafaxine in human blood serum
InactiveCN108982714AStrong specificityStrong specificity and high sensitivityComponent separationFluorescenceMedicine
The invention provides a simple and efficient method for detecting the concentration of venlafaxine and an active metabolite O-desmethylvenlafaxine in human blood serum and relates to the technical field of medicines. The simple and efficient method is finished through the following steps: (1) treating a blood serum sample; (2) preparing a solution; and (3) selecting chromatographic conditions. According to the simple and efficient method provided by the invention, a blood sample treatment process is optimized and the simple and efficient method is simple to operate and low in cost; a fluorescence method has strong specificity and high sensitivity; and a capability of accurately determining the venlafaxine and the active metabolite O-desmethylvenlafaxine is greatly improved and the reliability of the determination method is improved.
Owner:SHANGHAI MENTAL HEALTH CENT (SHANGHAI PSYCHOLOGICAL COUNSELLING TRAINING CENT)
Processes for the synthesis of O-Desmethylvenlafaxine
InactiveUS20090137846A1Organic compound preparationAmino compound preparationMedicinal chemistryO desmethylvenlafaxine
Owner:TEVA PHARM USA INC
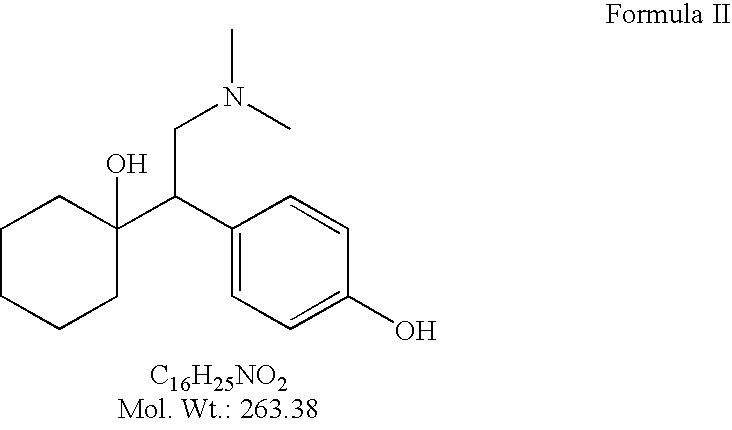
O-desmethylvenlafaxine
InactiveUS20100210719A1Easy to operateCost-effectiveBiocidePowder deliveryDesvenlafaxineOrganic chemistry
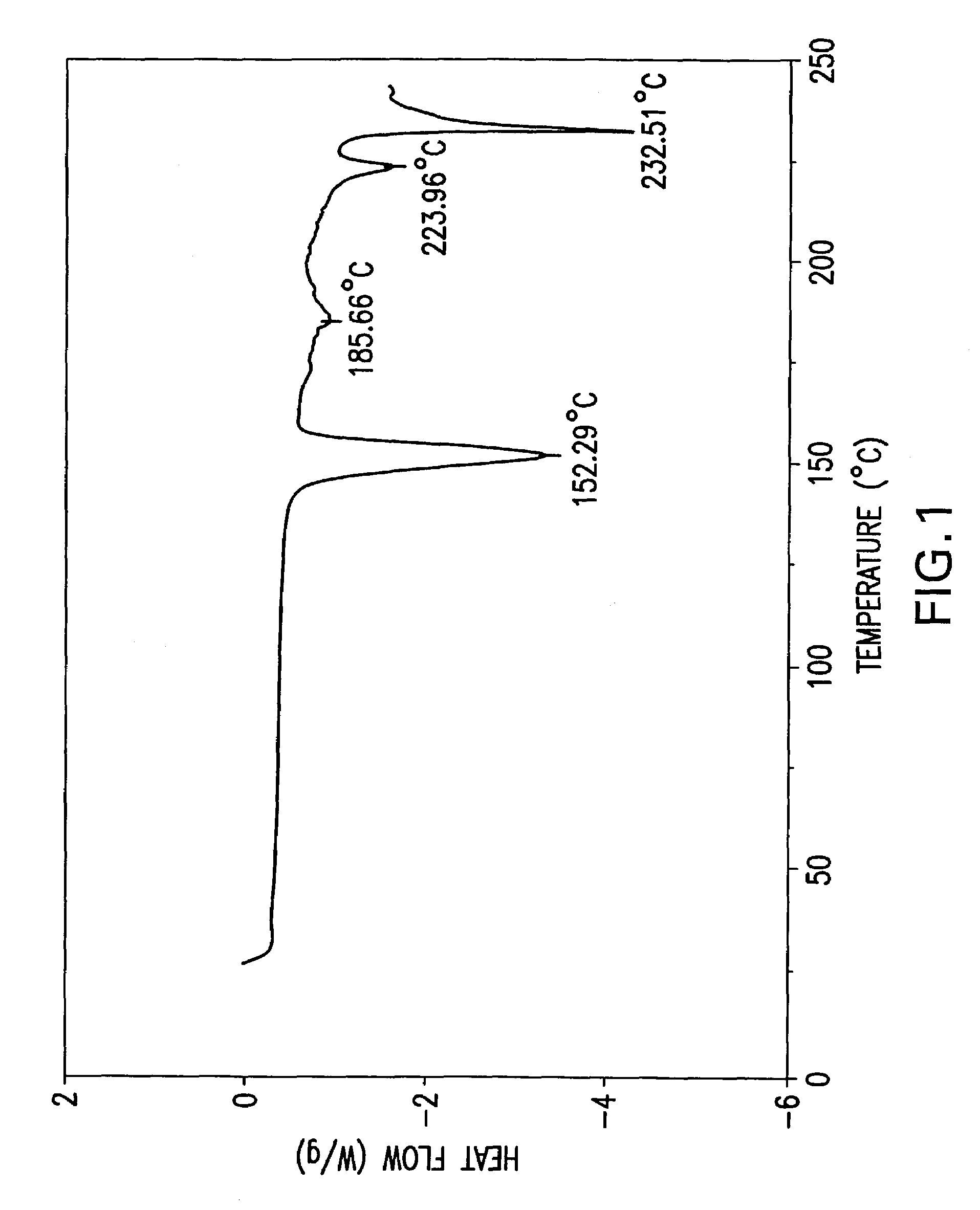
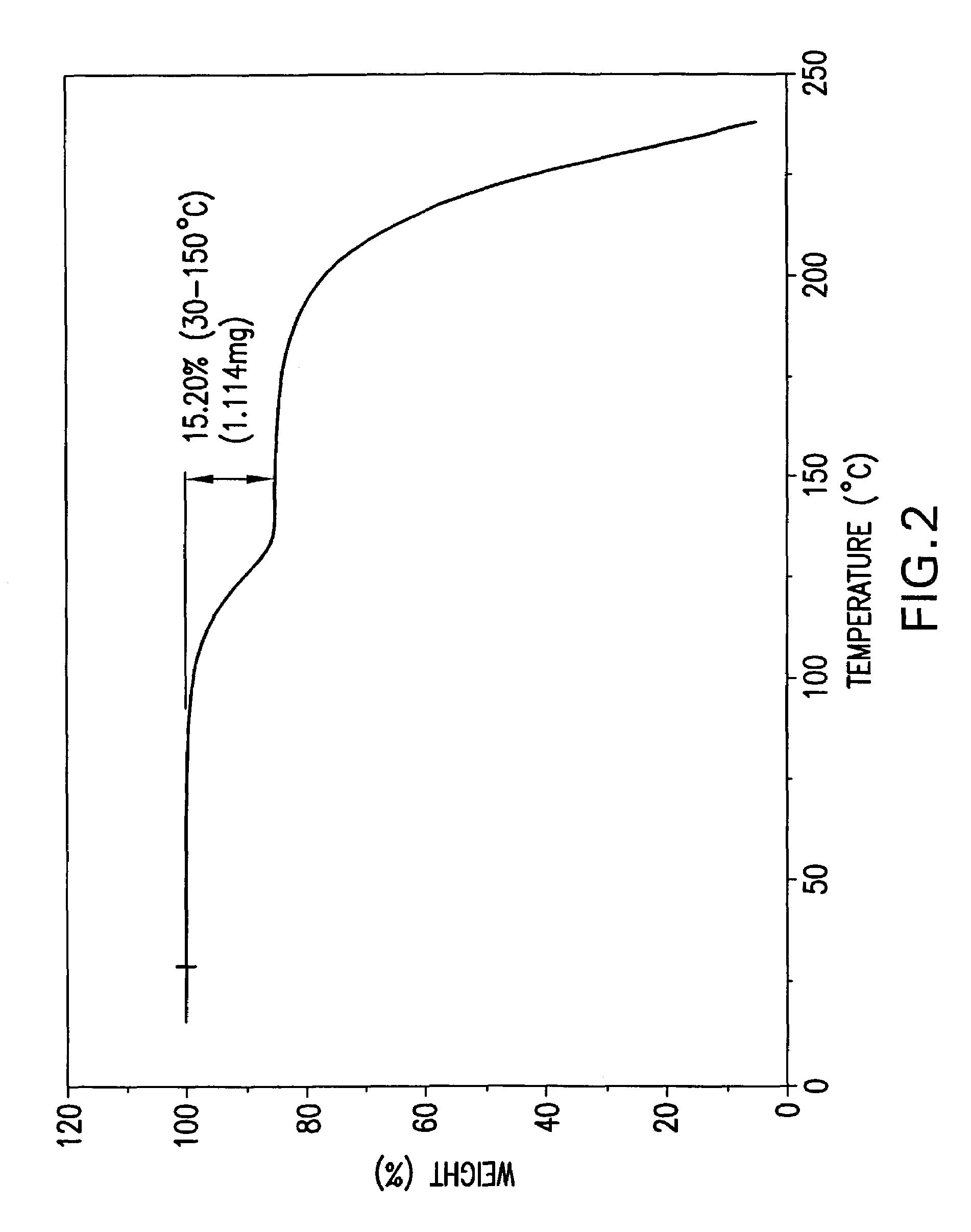
Processes for preparing desvenlafaxine and stable amorphous O-desmethylvenlafaxine succinate solid dispersions with one or more pharmaceutically acceptable carriers.
Owner:DR REDDYS LAB LTD +1
Processes for the preparation of odesmethylvenlafaxine, free from its dimer impurities
Owner:TEVA PHARM USA INC
Process for the preparation of O-desmethylvenlafaxine
InactiveCN102164886AEasy to handleImprove compatibilityOrganic active ingredientsNervous disorderThioureaMedicinal chemistry
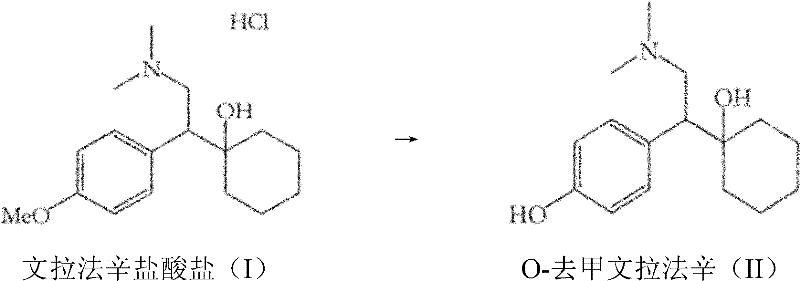
The present invention provides a convenient and efficient process for the preparation of O-desmethylvenlafaxine (ODV) or a salt thereof, comprising the reaction of venlafaxine, or a salt thereof, with a thiourea or a mixture of thioureas.
Owner:GENERICS UK LTD
Substantially pure o-desmethylvenlafaxine and processes for preparing it
Owner:TEVA PHARMA IND LTD
Substantially pure O-desmethylvenlafaxine and processes for preparing it
Owner:TEVA PHARM USA INC
Process for preparing O-desmethylvenlafaxine
InactiveCN101952240AEasy to handleHigh yieldOrganic active ingredientsNervous disorderAlcoholThiol Reagents
The present invention provides a convenient and efficient process for the preparation of O-desmethylvenlafaxine (ODV), comprising the reaction of venlafaxine, or a salt thereof, with a thiol reagent such as a dithiol, an aminothiol or an inorganic thiol. The present invention also provides a process for purifying ODV base,said process comprising the steps of mixing crude ODV base with an alcohol to form a suspension and adding acid followed by base to generate ODV base with high purity.
Owner:GENERICS UK LTD
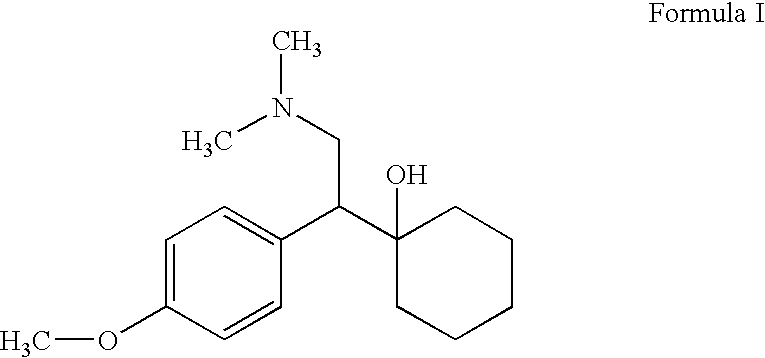
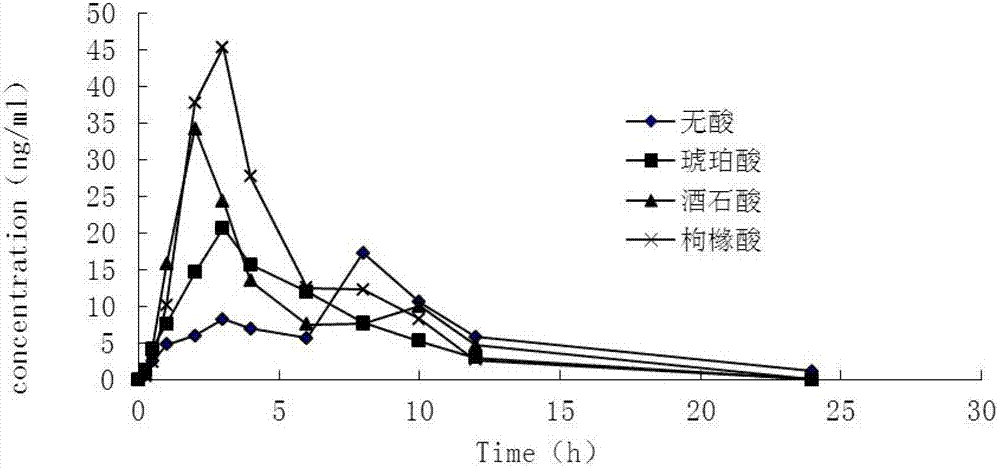
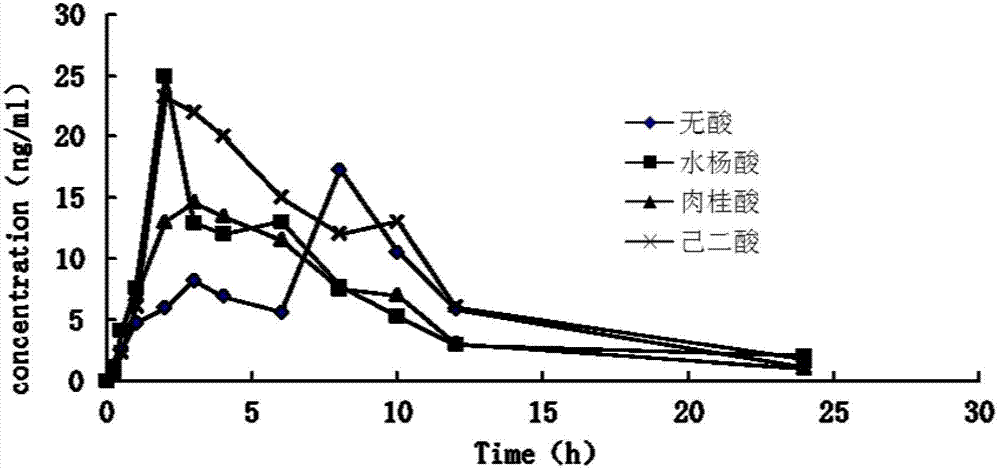
Controlled-release medicinal composition containing demethyl venlafaxine benzoate compounds
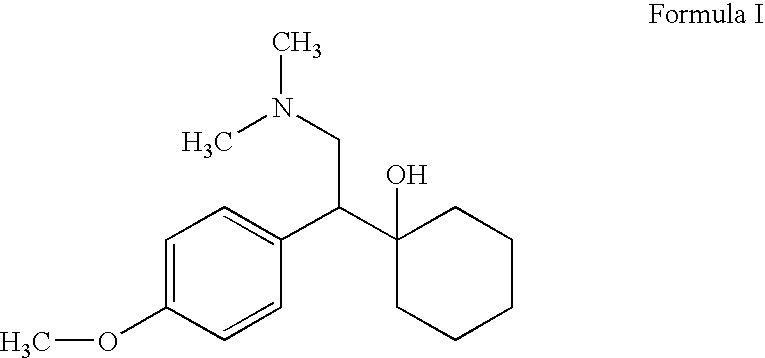
The invention provides a controlled-release medicinal composition containing demethyl venlafaxine benzoate compounds which contain a compound represented by a general formula (I), an acidity regulator and a controlled-release framework material. The ontrolled-release medicinal composition provided by the invention can remarkably improve oral bioavailability of the demethyl venlafaxine benzoate compound preparations, and a preparation technology is simple and convenient, and an effect on improving the bioavailability of preparation can be reached, and active compounds are not needed to be additionally preprocessed.
Owner:SHANDONG LUYE PHARMA CO LTD
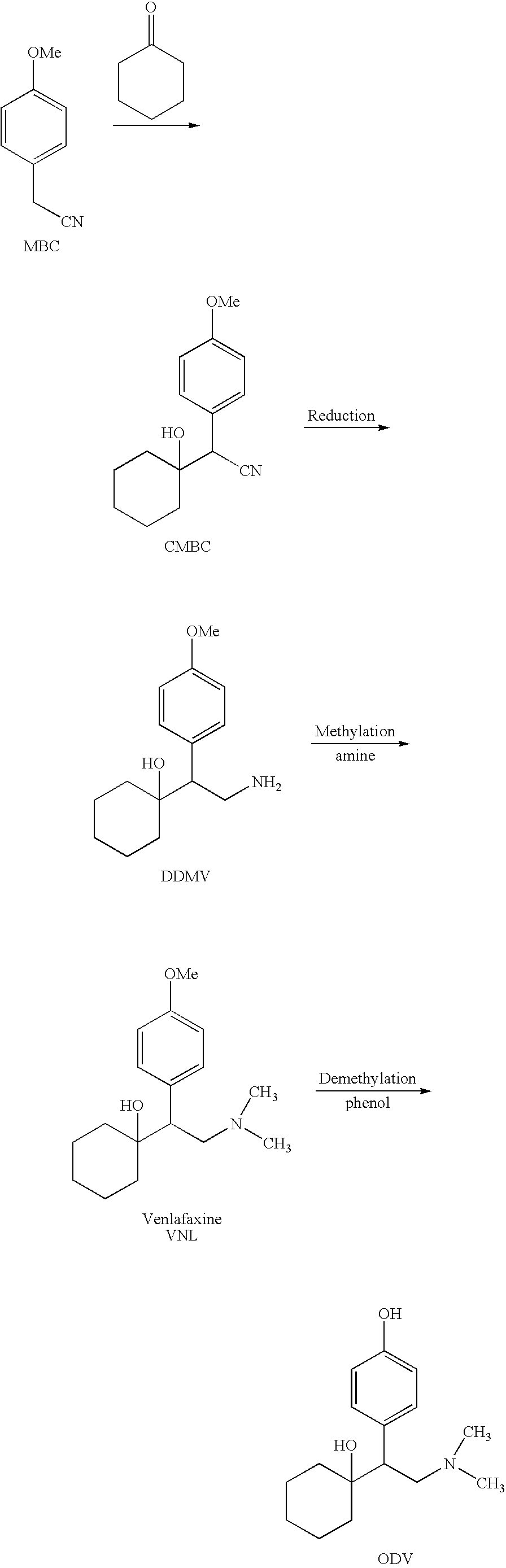
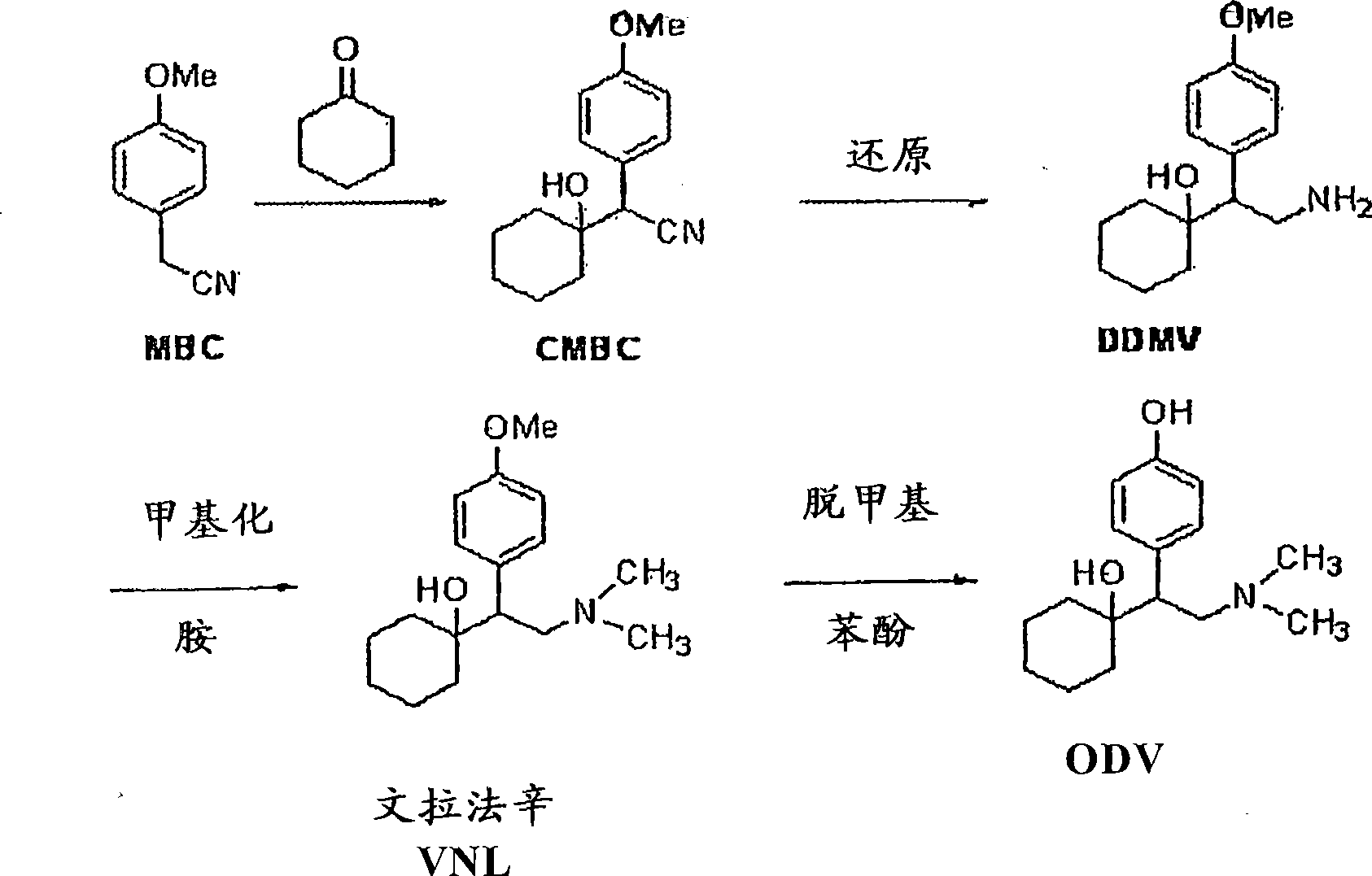
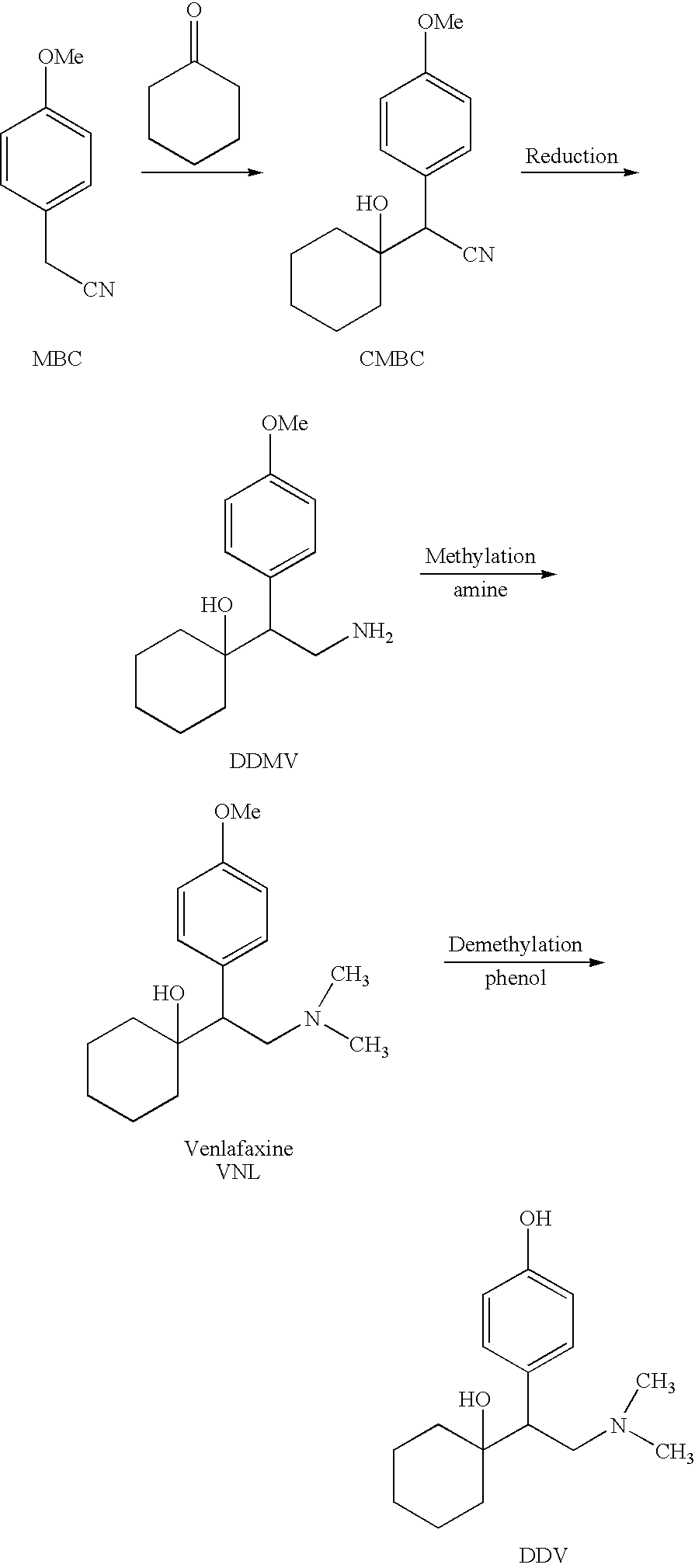
Preparation method of O-desmethylvenlafaxine
InactiveCN101781221AReduce dosageReduce pollutionOrganic compound preparationAmino-hyroxy compound preparationCyclohexanoneN dimethylformamide
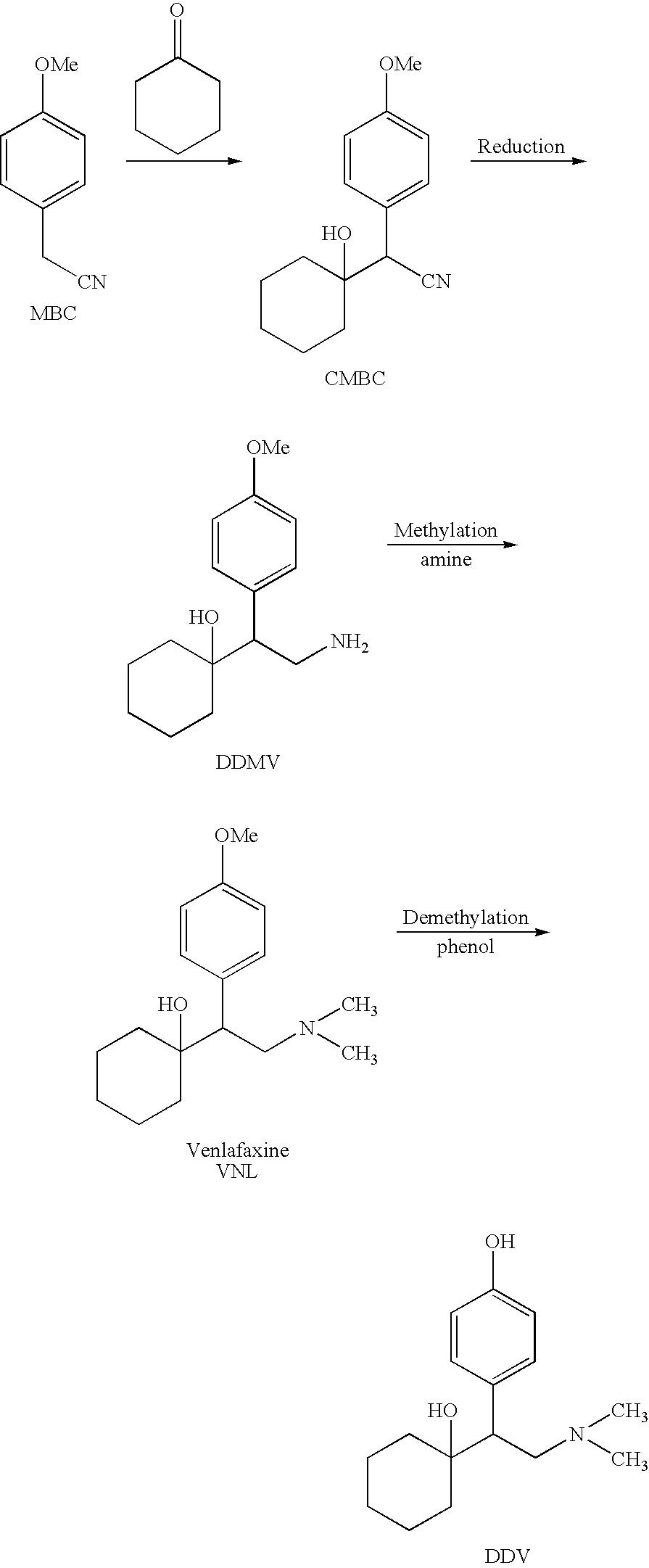
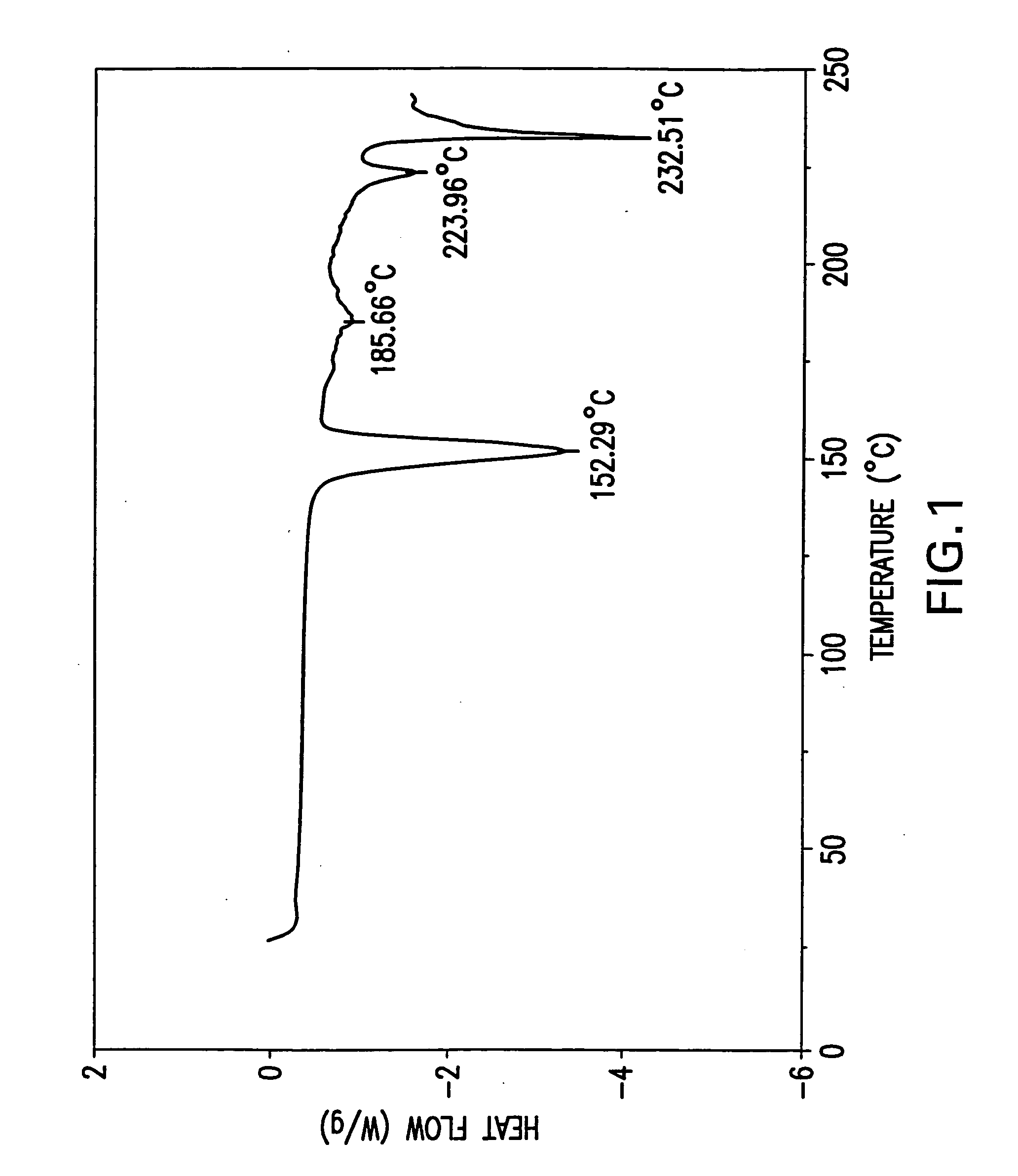
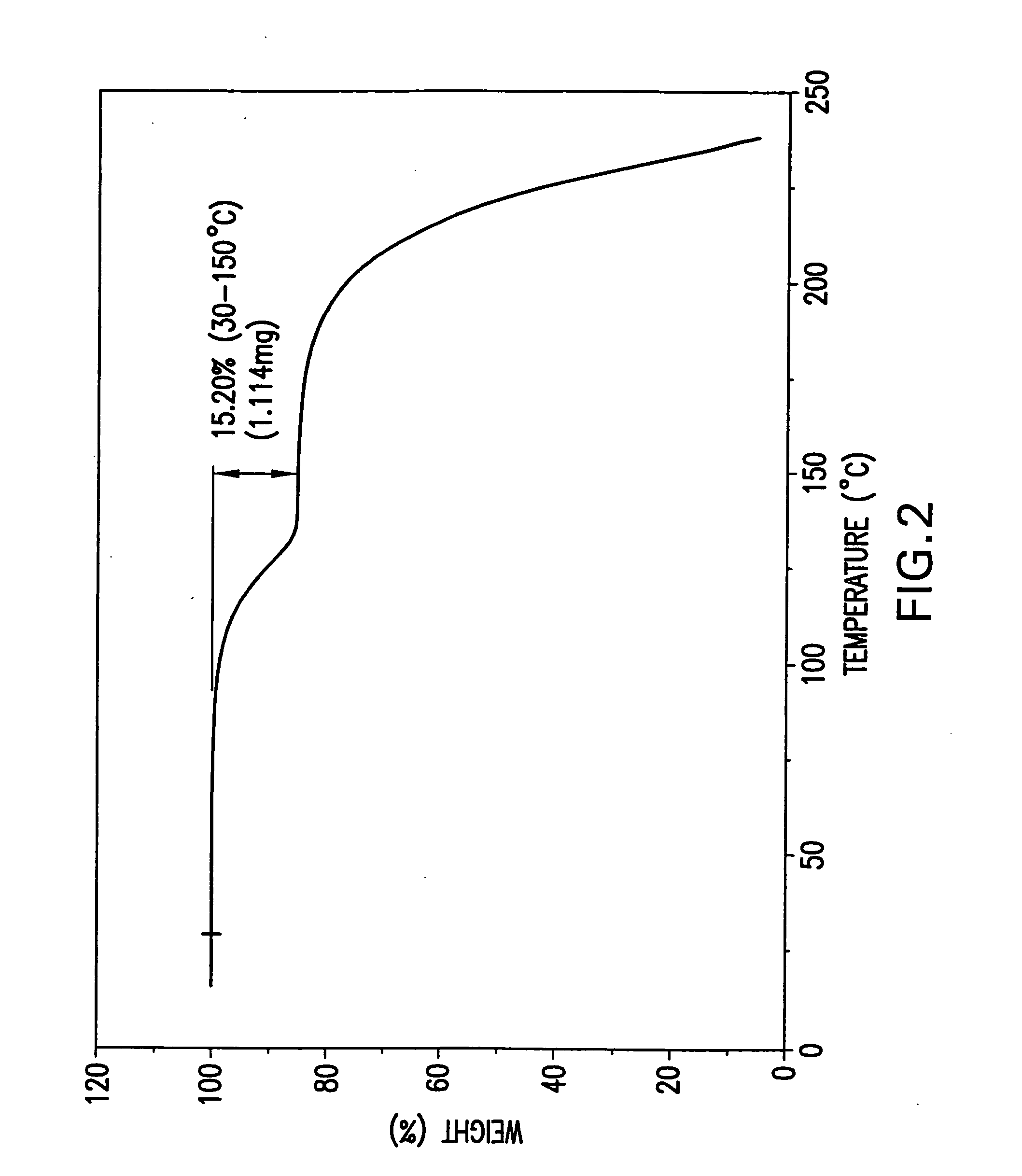
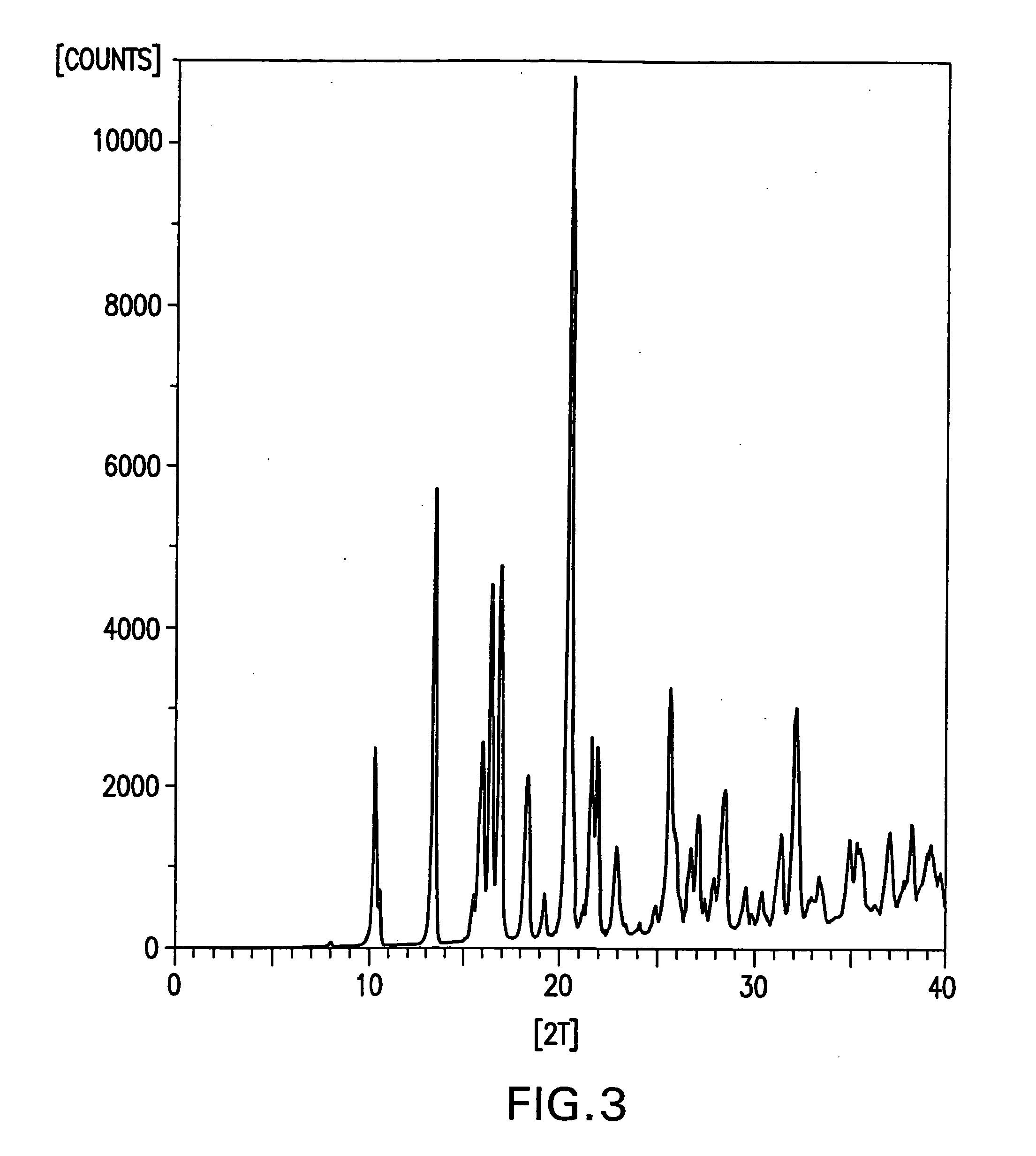
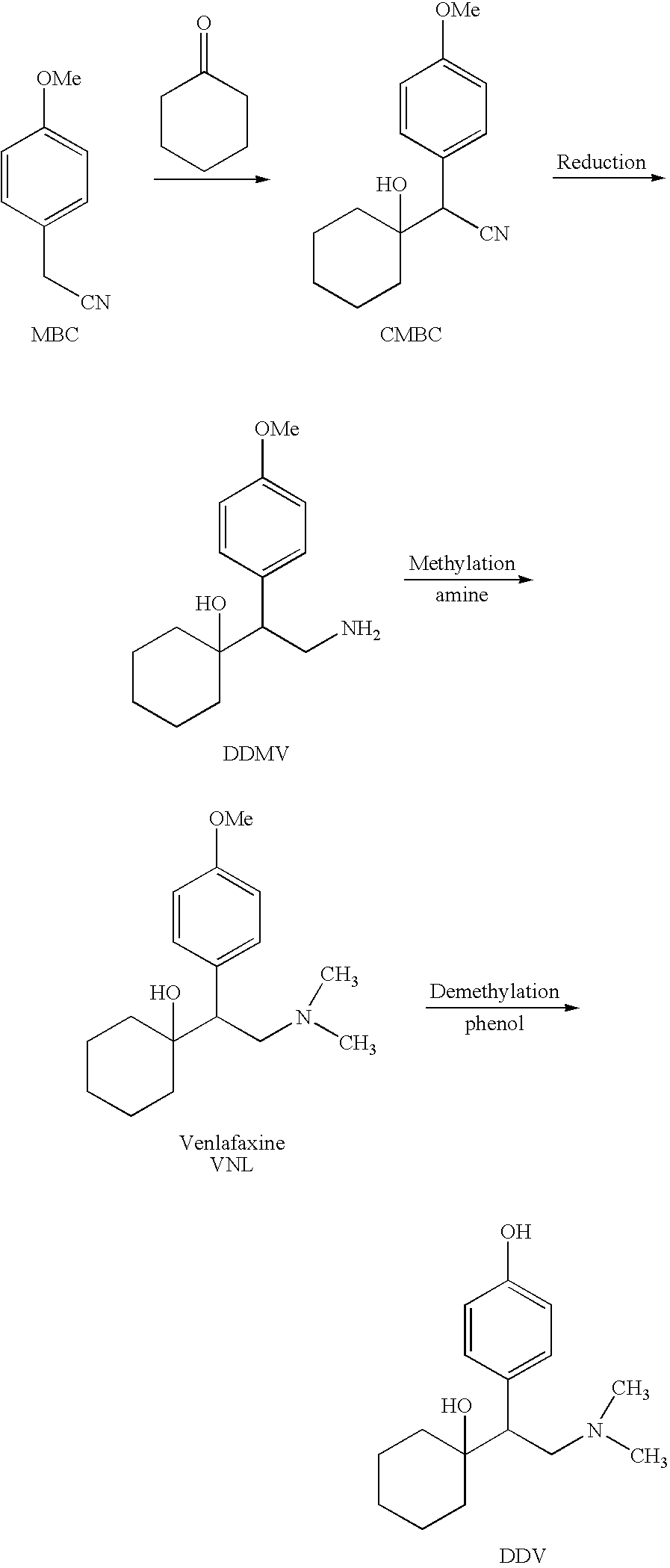
The invention relates to a preparation method of O-desmethylvenlafaxine. The method comprises the following special steps: 1. performing chlorination to p-hydroxyphenylacetic acid in aprotic organic solvent at room temperature, then reacting with dimethylamine aqueous solution to prepare 2-(4-hydroxyphenyl)-N,N-dimethylformamide; 2. using 2-(4-hydroxyphenyl)-N,N-dimethylformamide and cyclohexanone to perform condensation reaction at 0 to -78 DEG C under the action of butyl lithium and prepare 2-(1-hdroxycyclohexyl)-2-(4-hydroxyphenyl)-N,N-dimethylformamide; and 3. using lithium aluminium hydride to reduce 2-(1-hdroxycyclohexyl)-2-(4-hydroxyphenyl)-N,N-dimethylformamide. The preparation method of O-desmethylvenlafaxine provided by the invention is characterized in that the raw materials are accessible, the entire preparation contains only three steps, the preparation processes are simplified, the reaction conditions are mild, the operation is convenient and safe and the yield is high.
Owner:CHEMINNO SHANGHAI
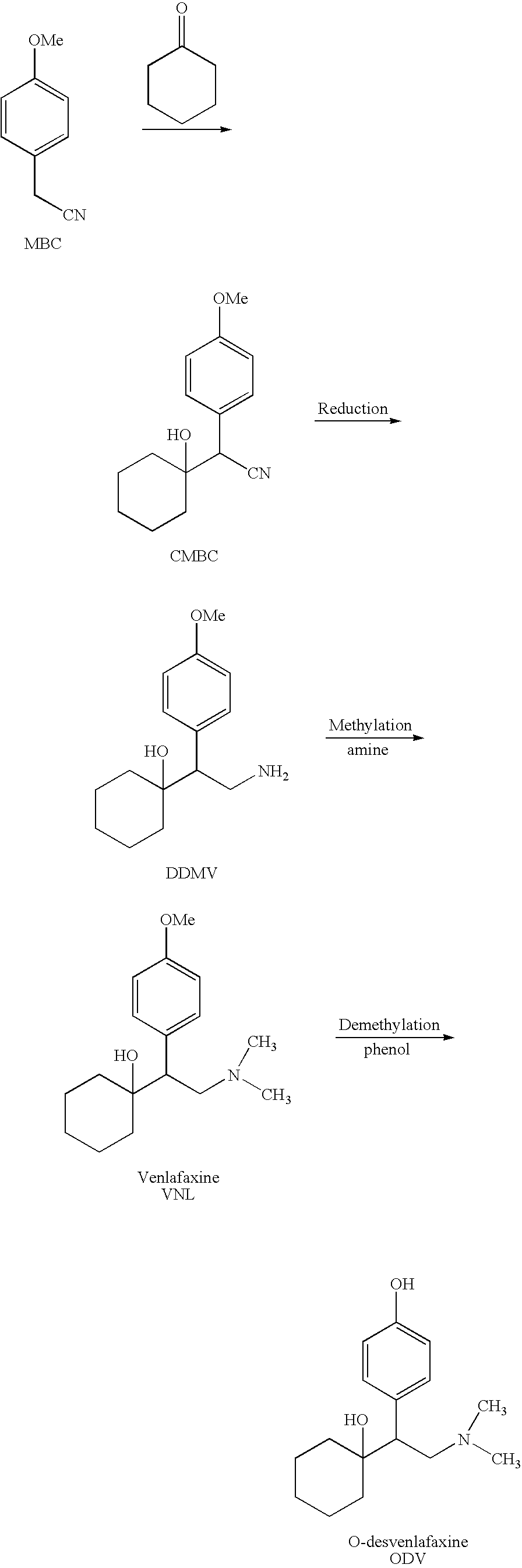
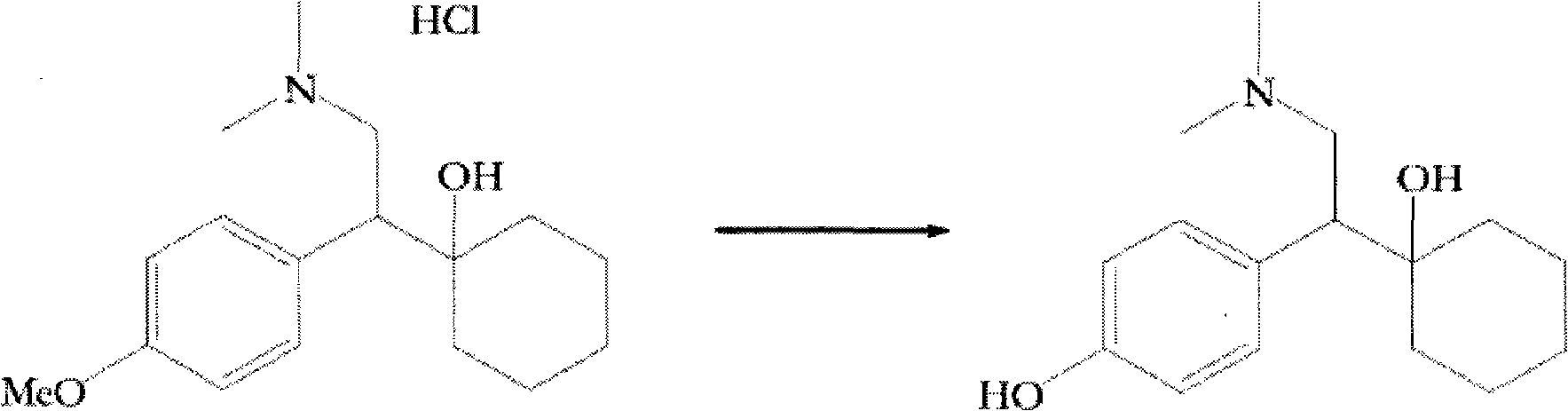
Novel method for preparing O-desvenlafaxine
ActiveCN101117320AHigh purityHigh yieldOrganic compound preparationAmino-hyroxy compound preparationMethylene DichlorideDesvenlafaxine
The invention provides a new method for preparation ODV, which comprises the following procedures:1. the compound I(1-[2-amino-1-(4- methoxybenzyl)ethyl] cyclohexanol) is made a demethylation reaction, the adopted catalyzer chooses from mercaptide anion, nickel, cobalt, metal sulphide, diphenylphosphine lithium or trialky borohydrid, the product is further purified to obtain the high pure compound II (1-[2-amino-1-(4-hydroxyphenyl)ethyl] cyclohexanol; 2. the compound II is included by the inclusion agent and is made a N, N-dimethylation reaction with the methylation reagent, the PH value is adjusted to precipitate a large quantity of solids (namely ODV), the inclusion agent is alpha- cyclodextrin hydrate, beta- cyclodextrin hydrate or gamma cyclodextrin hydrate; 3. the precipitated solids are dissolved by the methylene dichloride, are evaporized and are crystallized by the isopropanol. The invention has simple and quick operation, single preparation products, high yields, high purity, and greatly decreased production cost.
Owner:ZHEJIANG JIUZHOU PHARM CO LTD
Process for the synthesis of O-desmethylvenlafaxine
InactiveUS20080183016A1Nervous disorderOrganic compound preparationPhotochemistryO desmethylvenlafaxine
Owner:TEVA PHARM USA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com