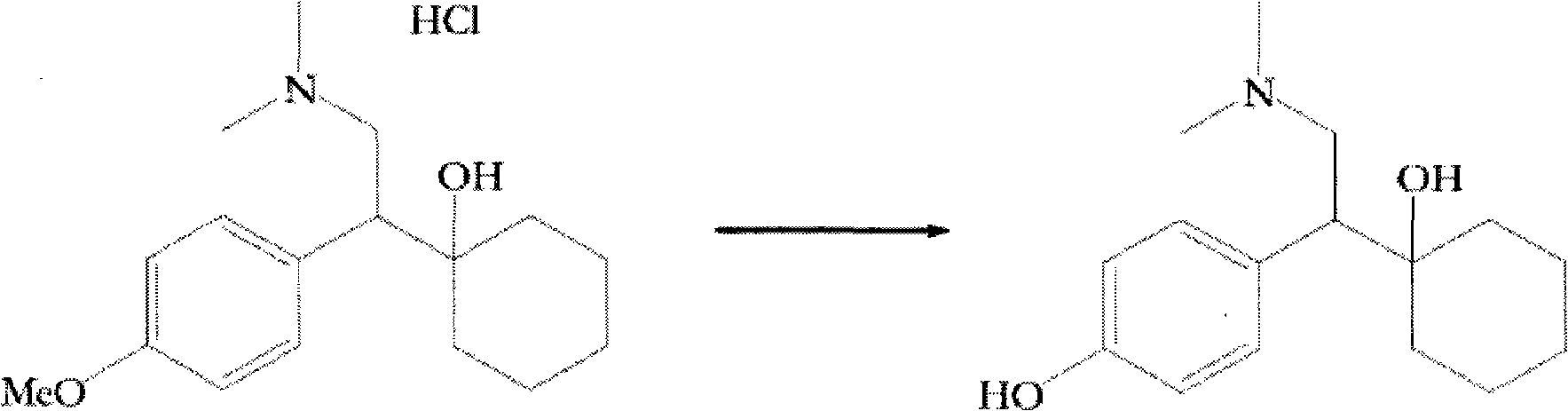
Process for preparing O-desmethylvenlafaxine
A technology of methyl and aryl groups, applied in the field of preparing O-desmethyl venlafaxine, can solve the problems of inconvenient long-term reaction time, high temperature, long process and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Preparation of ODV base from venlafaxine base using 1,2-ethanedithiol:
[0112] Add 1,2-ethanedithiol (10.17 g, 0.11 mol) to a suspension of potassium tert-butoxide (30.29 g, 0.27 mol) in polyethylene glycol 400 (125 mL) at 25°C-30°C middle. To this stirred suspension was added venlafaxine base (25 g, 0.09 mol) and the reaction mixture was heated to 130°C-135°C for 24-28 hours. After the reaction was complete, the reaction mixture was cooled to 25°C-30°C and water (500 mL) was added, followed by concentrated hydrochloric acid (35-37% w / v, 30 mL). The solution was extracted with toluene (2 x 50 mL). To this aqueous solution was added 25% w / v ammonia solution (35 mL) to adjust the pH of the solution to >9.5. A solid precipitated and was filtered to provide crude ODV base. The resulting solid was further suspended in methanol (125 mL), and concentrated hydrochloric acid (35-37% w / v, 15 mL) was added to the suspension to dissolve the solid, followed by addition of 25% w...
Embodiment 2
[0114] Preparation of ODV base from venlafaxine hydrochloride using 1,2-ethanedithiol:
[0115] Add 1,2-ethanedithiol (10.17 g, 0.11 mol) to a suspension of potassium tert-butoxide (30.29 g, 0.27 mol) in polyethylene glycol 400 (125 mL) at 25°C-30°C middle. To this stirred suspension was added venlafaxine hydrochloride (28 g, 0.09 mol) and the reaction mixture was heated to 130°C-135°C for 24-28 hours. After the reaction was complete, the reaction mixture was cooled to 25°C-30°C, and water (500 mL) was added, followed by concentrated hydrochloric acid (35-37% w / v, 20 mL). The solution was extracted with toluene (2 x 50 mL). To this aqueous solution was added 25% w / v ammonia solution (25 mL) to adjust the pH of the solution to >9.5. A solid precipitated and was filtered to provide crude ODV base. The resulting solid was further suspended in methanol (140 mL), and concentrated hydrochloric acid (35-37% w / v, 17 mL) was added to the suspension to dissolve the solid, followed b...
Embodiment 3
[0117] Preparation of ODV base from venlafaxine base using 2-diethylaminoethanethiol:
[0118] Add 2-diethylaminoethanethiol hydrochloride (3.06 g, 0.018 mol) to a suspension of sodium methoxide (2.9 g, 0.054 mol) in polyethylene glycol 400 (50 mL) at 25°C-30°C in the liquid. To this stirred suspension was added venlafaxine base (2.5 g, 0.009 mol) and the reaction mixture was heated to 170°C-175°C for 24-28 hours. After the reaction was complete, the reaction mixture was cooled to 25°C-30°C, and water (200 mL) was added, followed by concentrated hydrochloric acid (35-37% w / v, 5 mL). The solution was extracted with toluene (2 x 25 mL). To this aqueous solution was added 25% w / v ammonia solution (7 mL) to adjust the pH of the solution to >9.5. A solid precipitated and was filtered to provide crude ODV base. The resulting solid was further suspended in methanol (12.5 mL), and concentrated hydrochloric acid (35-37% w / v, 7.5 mL) was added to the suspension to dissolve the solid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com