Highly bioavailable oral delayed release dosage forms of O-desmethylvenlafaxine succinate
A methyl venlafaxine, oral dosage technology, applied in the field of treating depression and reducing the side effects of O-desmethylvenlafaxine, can solve the problems of variable effects of hydrogel preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: 2% Surelease (Ethylcellulose Dispersion)
[0049] Element
[0050] * Not present in the final formulation
Embodiment 2
[0051] Example 2: 3% Surelease (Ethylcellulose Dispersion)
[0052] Element
[0053] Release coat:
[0054] * Not present in the final formulation
Embodiment 3
[0055] Example 3: Enteric Coated Capsules Containing Hypromellose / Microcrystalline Cellulose Spheroid Cores
[0056] Element
mg / capsule (150mgODV dose)
Small ball core:
DVS-233
227.62
microcrystalline cellulose
97.55
Hypromellose
65.0
Sealing layer:
Opadry Clear
6.50
Enteric coating:
Eudragit L30-D55
71.77
triethyl citrate
2.15
sodium hydroxide
3.23
Talc
10.64
water *
Not applicable
[0057] * Not present in the final formulation
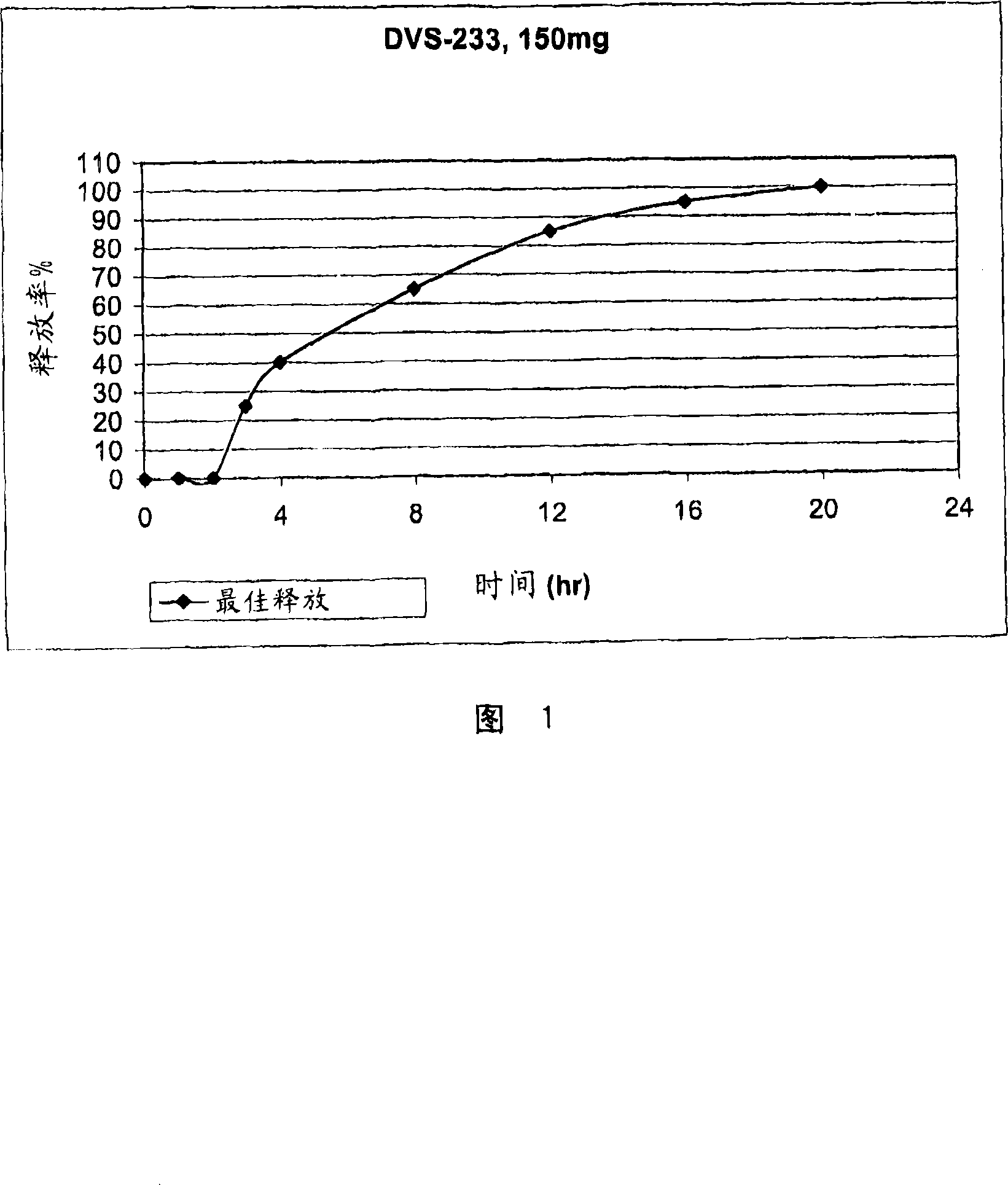
[0058] This formulation is expected to release in vivo more than 85% of its content within 12 hours after a 2 hour lag period following oral administration of the product, and to have approximately 100% release within 20 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com