Delayed-release glucocorticoid treatment of rheumatoid disease
a glucocorticoid and rheumatoid disease technology, applied in the direction of anti-inflammatory agents, muscular disorders, drug compositions, etc., can solve the problems of unacceptable adverse reactions, long-term use of high doses is associated with clinically significant side effects, and the response of the permanently stimulated hpa axis is inadequate, so as to achieve the effect of increasing the
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
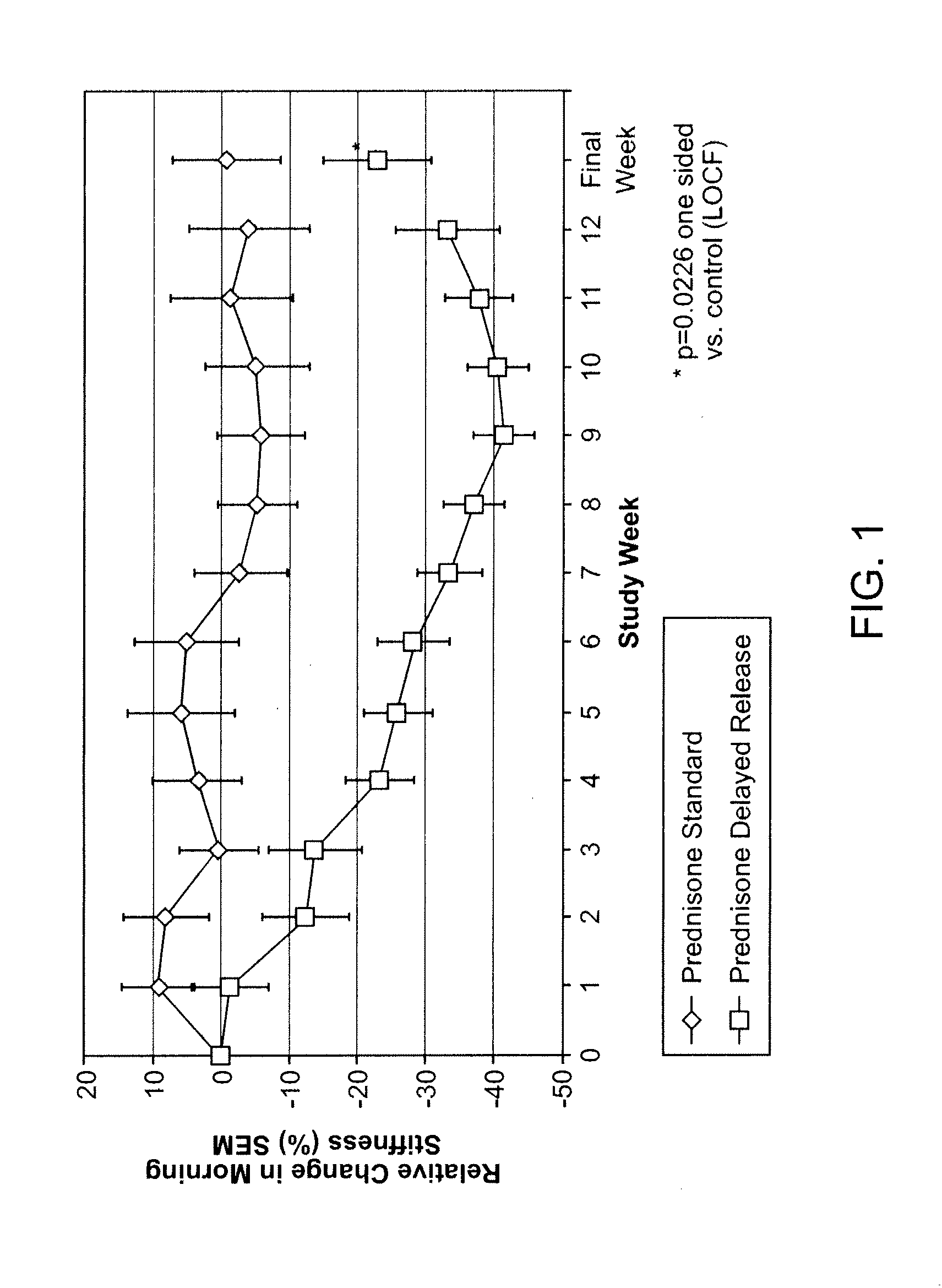
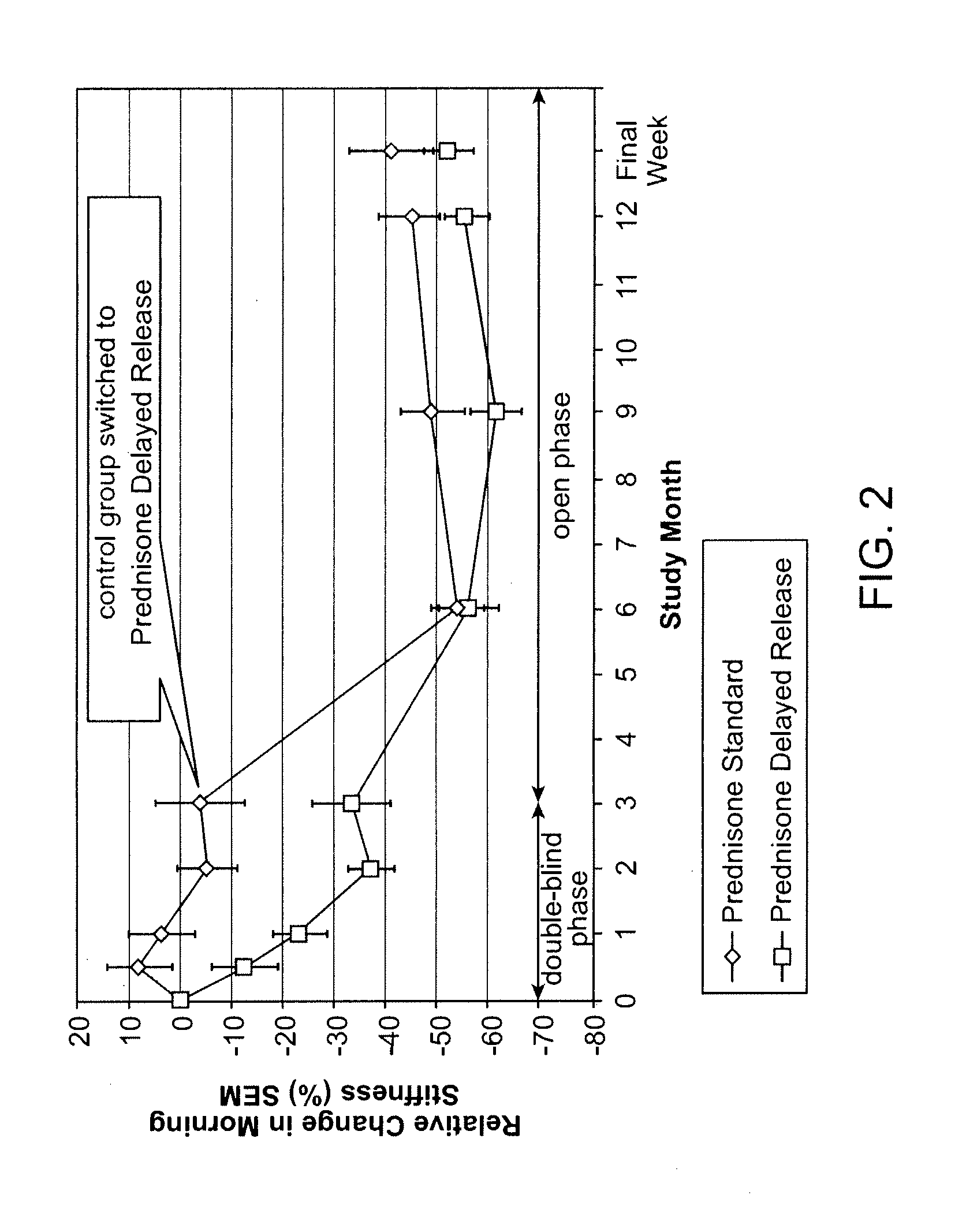
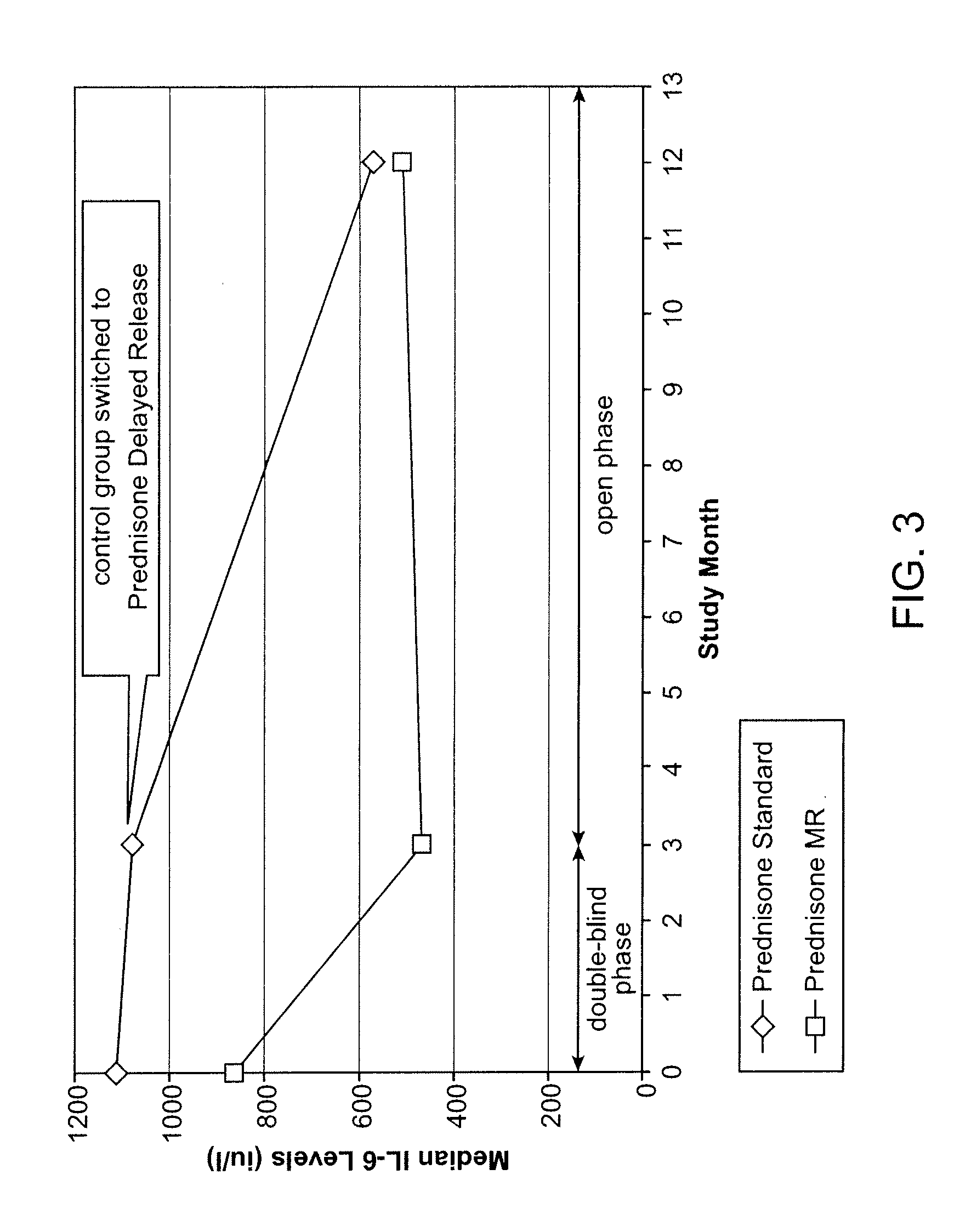
[0121]Clinical studies. The clinical development program supporting the present application for the delayed-release prednisone tablet “Prednisone delayed-release” comprised 3 phase I studies and 1 phase III study:[0122]Phase I studies: These 3 randomized, open-label, crossover studies on 69 healthy men investigated the comparative bioavailability and pharmacokinetic characteristics of 6 experimental galenic delayed-release formulations each containing 5 mg prednisone. The studies were performed to allow selection of a delayed-release tablet with appropriate characteristics for evening administration to RA patients (i.e. a suitable lag time and high bioavailability that was not affected by food). Single doses of each of the delayed-release tablets were compared to a single dose of a reference immediate release (IR) prednisone tablet (Decortin® 5 mg tablets marketed by Merck KGaA).[0123]Phase III study: In this randomized, parallel-group, double-blind, double-dummy study on 288 adult ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| lag time | aaaaa | aaaaa |
| lag time | aaaaa | aaaaa |
| dissolution time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com