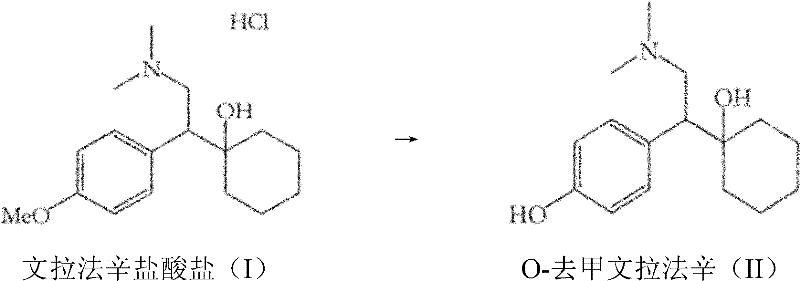
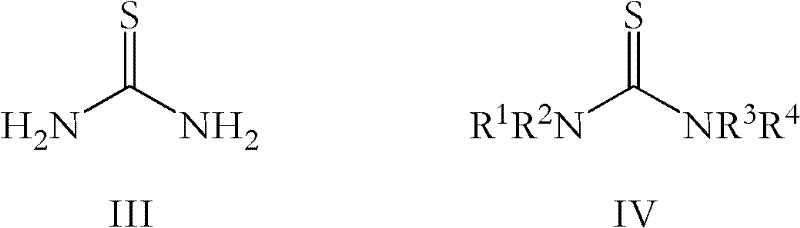Process for the preparation of O-desmethylvenlafaxine
A technology for desvenlafaxine and methanol, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of aminohydroxyl compounds, etc., and can solve problems such as long processing time, inconvenient needs, long reaction time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1: Preparation of ODV bases from venlafaxine bases using thiourea
[0085] Thiourea (13.7 g, 0.18 mol) was added to a suspension of potassium hydroxide (20.2 g, 0.36 mol) in polyethylene glycol 400 (50 mL) at 25-30°C. Venlafaxine base (10 g, 0.04 mol) was added to the suspension with stirring and the reaction mixture was heated to 170-180°C. After completion of the reaction (16-20 hours), the reaction mixture was cooled to 60-70° C., and water (40 mL) was added, followed by 35% aqueous hydrochloric acid (15-20 mL). The solution was washed with dichloromethane (2x50 mL). To this aqueous solution was added 25% aqueous ammonia to adjust the pH of the solution to >9.5. A solid precipitated out and was filtered to give crude ODV base. Additional crude ODV base was added to methanol (250 mL), refluxed for 1 h, then cooled to 10-15 °C. The crystallized pure ODV base was filtered off and dried at 50-55°C. Instead of crystallization in methanol, the crude ODV base ...
Embodiment 2
[0089] Example 2: Preparation of ODV base from venlafaxine hydrochloride using thiourea
[0090] Thiourea (12.1 g, 0.16 mol) was added to a suspension of potassium hydroxide (17.8 g, 0.32 mol) in polyethylene glycol 400 (50 mL) at 25-30°C. Venlafaxine hydrochloride (10.0 g, 0.03 mol) was added to the suspension with stirring, and the reaction mixture was heated to 170-180°C. After the reaction was complete (16-20 hours), the reaction mixture was cooled to 60-70°C and water (40 mL) was added, followed by 35% aqueous hydrochloric acid (15-20 mL). The solution was washed with dichloromethane (2x50 mL). To this aqueous solution was added 25% aqueous ammonia to adjust the pH of the solution to >9.5. A solid precipitated out and was filtered to give crude ODV base. The crude ODV base was crystallized in methanol and dried to give pure ODV base as an off-white solid. Instead of crystallization in methanol, the crude ODV base was purified by acid / base purification in methanol by...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com