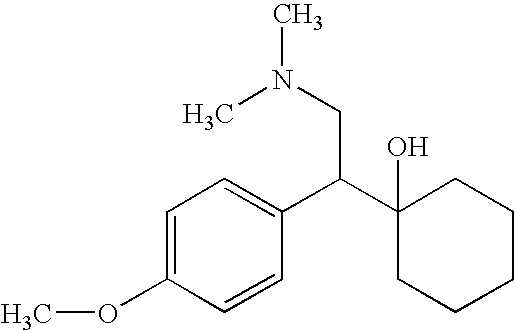
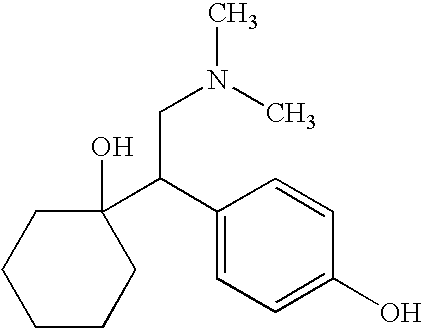
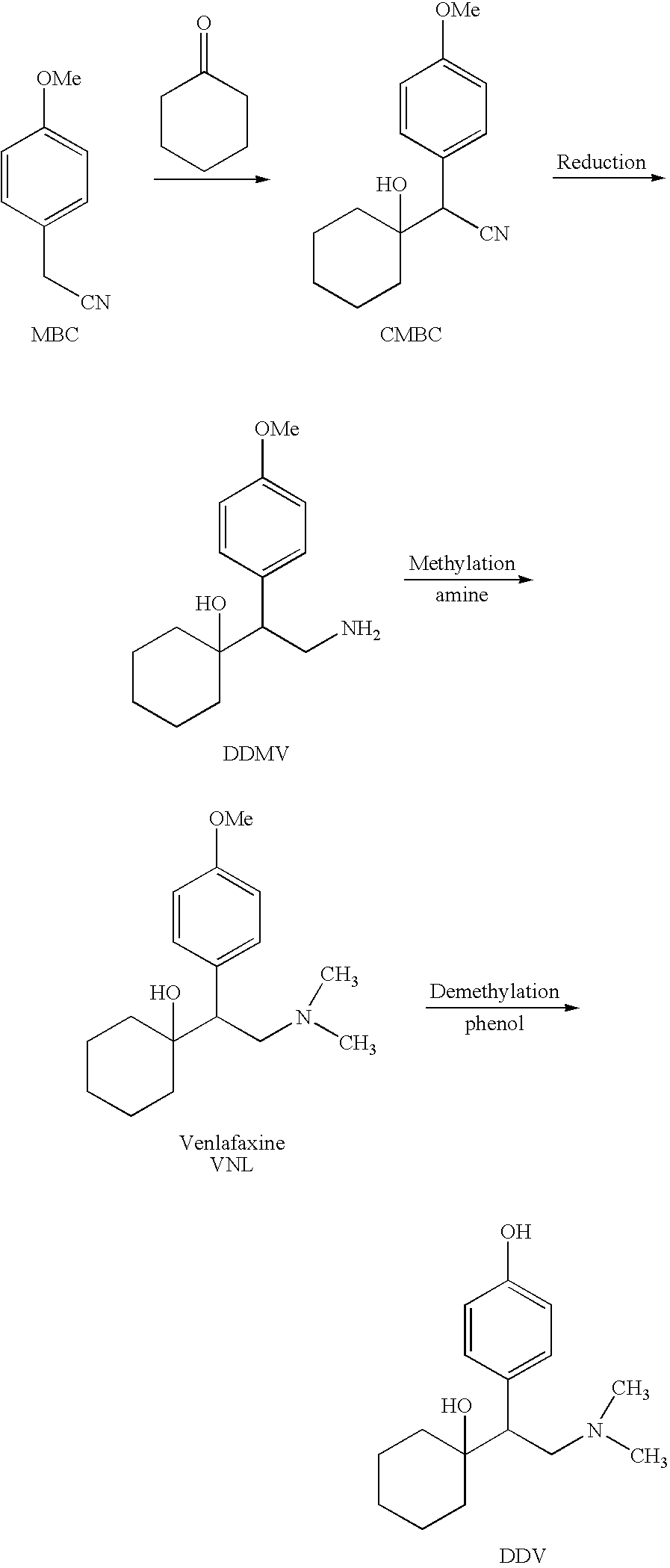
Process for the synthesis of O-desmethylvenlafaxine
a technology of odesmethylvenlafaxine and synthesis process, which is applied in the field of synthesis of odesmethylvenlafaxine, can solve the problems of long operation time, complex operation, and inability to meet industrial scale manufacturing requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0081]A 500 ml three-neck flask equipped with nitrogen inlet, thermometer and mechanical stirrer was charged with OBcarboxy (10 g, 65.72 mmol), DMF (1 ml) and CH2Cl2 (50 ml). The reaction mixture was stirred at 0° C. and SOCl2 was added dropwise.
[0082]The reaction was stirred at ambient temperature for 2 hours and then the solvent was evaporated under reduced pressure. The residue was dissolved in CH2Cl2 (50 ml) and dimethylamine-HCl (100 g, 1.22 mol) was added. Then diisopropylethylamine (150 ml, 0.882 mol) was added dropwise. The mixture was stirred at ambient temperature overnight and then washed with a saturated solution of NaHCO3; a precipitate appeared. The precipitate was filtered under reduced pressure and washed with methylene chloride. The solid so-obtained was dried in a vacuum oven at room temperature to get 5.55 g of OBA (purity 99.45%).
[0083]The organic layer was washed with brine and evaporated to dryness yielding crystals 5.84 g OBA (purity 96.57%). Total yield=97.85...
example 2
[0084]A 100 ml three-neck flask equipped with nitrogen inlet, thermometer and mechanical stirrer was charged with OBA (2.4 g, 13.39 mmol) TBDMS-Cl (4.5 g, 29.9 mmol), imidazole (5.5 g, 80.78 mmol) and CH2Cl2 (20 ml). The reaction mixture was stirred at ambient temperature for 2 hours. The reaction was quenched with brine and a 10% aqueous solution of citric acid The organic phase was then washed with brine and dried over Na2SO4. After filtration the solvent was evaporated under reduced pressure to get 3.82 g OBA-P (purity: 99.34%, yield: 97.45%).
example 3
[0085]In a 50 ml flask equipped with a mechanical stirrer, OBA (1.45 g, 8.09 mmol) was dissolved at room temperature in DHP (8 ml) under nitrogen. Pyridinium p-toluene sulfonate (PPTS, catalytic amount) was added and the reaction mixture was heated to 55° C. for 5 hours. The reaction was monitored by HPLC. EtOAc was added and the organic layer was washed with brine, dried over MgSO4 and filtered under reduced pressure to get OBA-DHP.
Preparation of Protected COBA (PCOBA)
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com