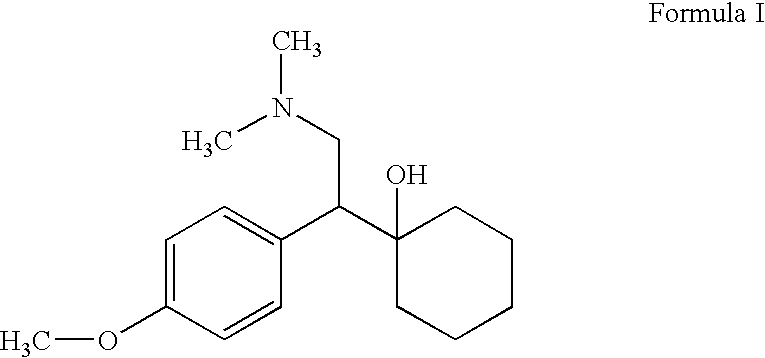
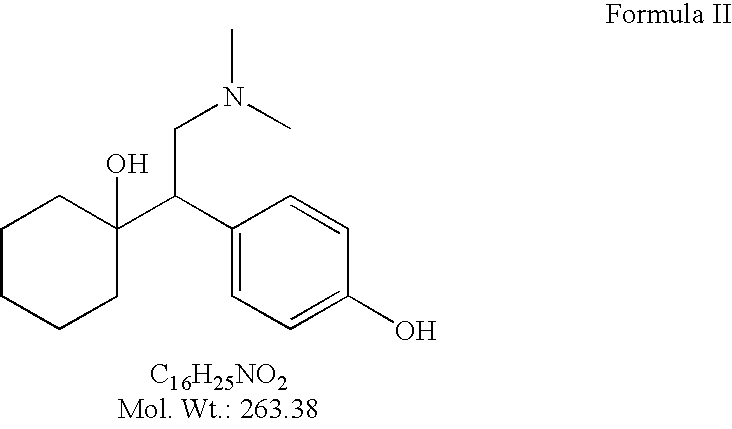
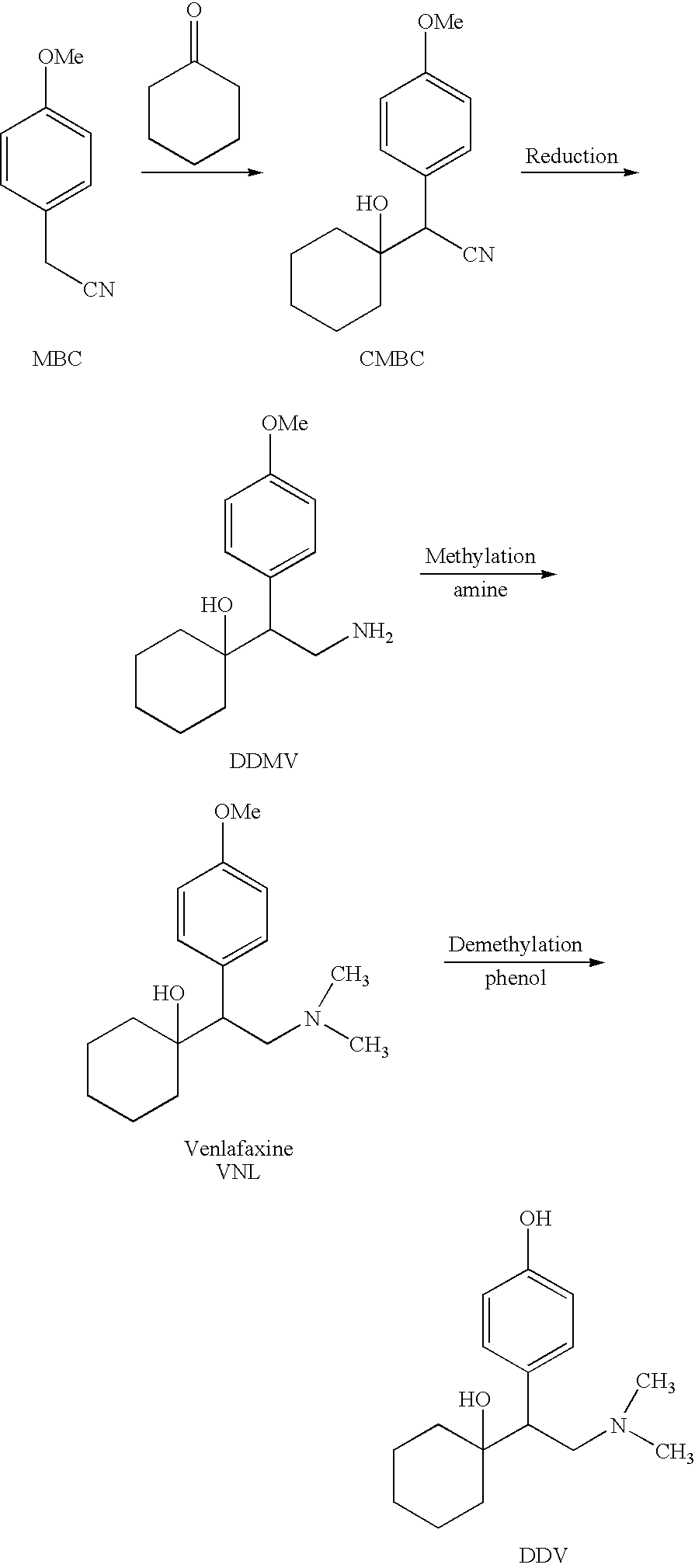
Substantially pure O-desmethylvenlafaxine and processes for preparing it
a technology of odesmethylvenlafaxine and odesmethylvenlafaxine, which is applied in the field of substantial pure odesmethylvenlafaxine, can solve problems such as harm to patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of O-Desmethylvenlafaxine in NMP
[0104] Venlafaxine (50 g, 180 mmol), thiophenol (20 ml, 195 mmol), K2CO3 (1 g, 6 mmol), and NMP (90 ml) were charged in a 500 ml 3 necks flask equipped with stirrer, condenser and thermometer. The mixture was heated to 190° C. After 5 hours at 190° C. the heating bath was removed. (less than 1.5% VNL). At 80° C. IPA (300 ml) was added. The solution was cooled to 0-5° C. overnight. The solid was filtered under reduced pressure and washed with IPA and water. The solid was then dried overnight at 50° C. under vacuum to get pure ODV base. ODV was obtained with a purity of 97% and an Assay of 93.5%.
example 2
Preparation of O-Desmethylvenlafaxine in NMP
[0105] To one neck flask equipped with magnetic stirrer, dean stark, condenser and thermometer were added at room temperature under flow of nitrogen VNL (5 g, 18.2 mmol), Na2S Hydrate (1.58 g, 12 mmol, assay >60%) and NMP (12 ml). The reaction mixture was heated to 150° C. in 1 hour and kept at this temperature for 7.5 hours. Then the reaction mixture was cooled to room temperature and stirred overnight at this temperature. Afterwards Na2S hydrate (0.71 g, 5.4 mmol, assay >60%) was added. The mixture was heated to 165° C. in 1 hour and kept at this temperature for 5 hours. After this time the reaction was cooled to 40° C., EPA (30 ml) and a 10% aqueous solution of citric acid (20 ml) were added slowly through a dropping funnel until light precipitation was observed (pH 10). The suspension was stirred over weekend at room temperature and the solid was filtered under reduced pressure and washed with IPA (20 ml). The solid was dried overnigh...
example 3
Preparation of O-Desmethylvenlafaxine Under Pressure
[0106] A 250 ml autoclave is charged with 5 g VNL (0.0182 mol), 3.81 g Sodium Ethanethiolate (0.0458 mol, 2.5 eq) and NMP (10 ml). The reaction mixture is stirred from 30° C. to 220° C. and 1-20 bar pressure for 4 h. The mixture is then cooled to room temperature. At ambient temperature IPA (10 ml) and water (10 ml) are added. To this mixture a 10% aqueous solution of citric acid is added in order to reach pH about 12. A solid begins to precipitate and is stirred at RT for 2.5 h. The solid is then filtered under reduced pressure and washed with solvent. The wet cake is dried in a vacuum oven at 50° C. to obtain pure ODV.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com