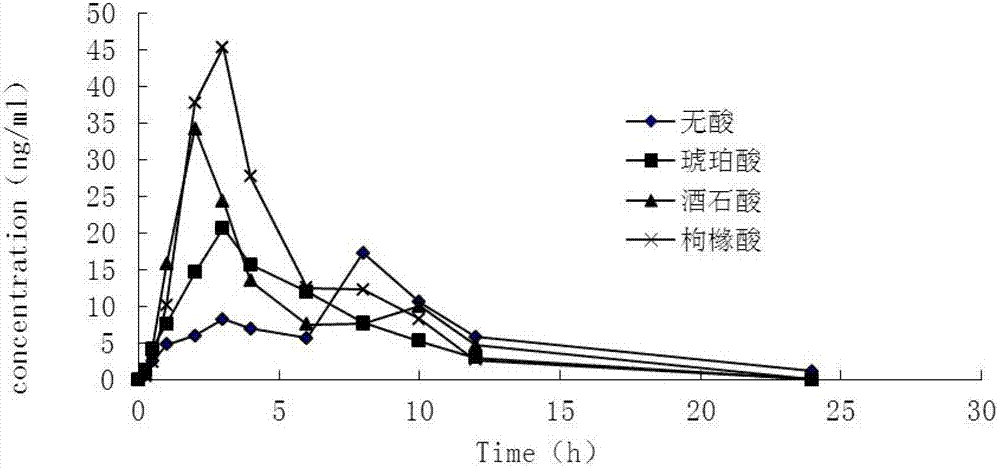
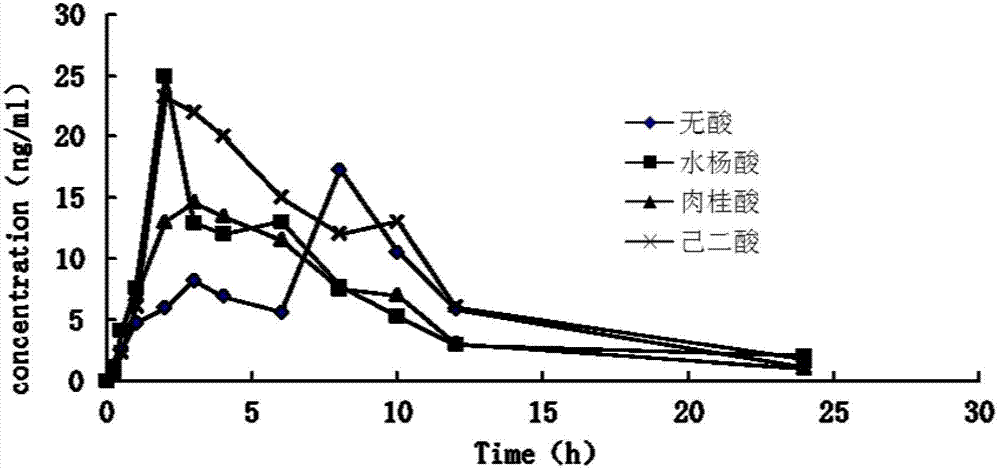
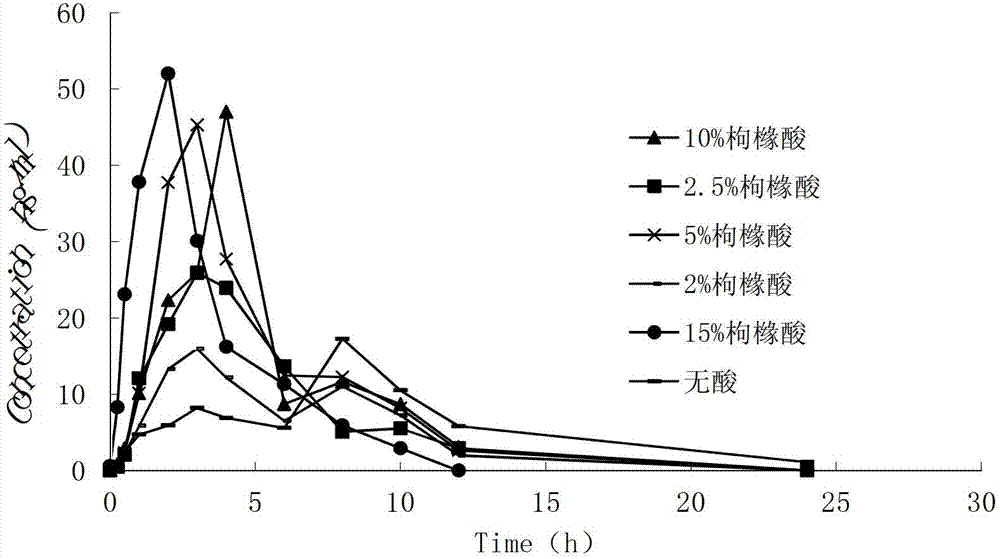
Controlled-release medicinal composition containing demethyl venlafaxine benzoate compounds
A sustained-release drug and composition technology, applied in the field of sustained-release pharmaceutical composition and its preparation, can solve the problems of low bioavailability of preparations, unfavorable preparation development, and reduced solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Prescription: 4-[2-dimethylamino-1-(1-hydroxycyclohexyl)ethyl]phenyl 4-methylbenzoic acid hydrochloride 42g, HPMC K4M CR 112g, citric acid 7g, microcrystalline Cellulose 187.25g, magnesium stearate 1.75g
[0029] Preparation method: The raw and auxiliary materials are all passed through an 80-mesh sieve for later use; weigh 4-[2-dimethylamino-1-(1-hydroxycyclohexyl)ethyl]phenyl hydrochloride of 4-methylbenzoate according to the prescription Salt, hydroxypropyl methylcellulose, microcrystalline cellulose, citric acid, mixed evenly; dry granulation machine granulation; adding magnesium stearate, mixed evenly and pressed into tablets, tablet weight 350±15mg, hardness 6±1kg, Make 1000 pieces, that is.
Embodiment 2
[0031] Prescription: 4-[2-dimethylamino-1-(1-hydroxycyclohexyl)ethyl]phenyl 4-methylbenzoic acid hydrochloride 112g, HPMC K4M CR112g, citric acid 8.75g, microcrystalline Cellulose 106.75g, 5% PVP absolute ethanol solution 140ml, magnesium stearate 3.5g
[0032] Preparation method: Weigh 4-[2-dimethylamino-1-(1-hydroxycyclohexyl)ethyl]phenyl hydrochloride, hydroxypropyl methylcellulose, Mix microcrystalline cellulose and citric acid evenly; add an appropriate amount of 5% PVP ethanol solution to the above mixed powder to prepare a soft material, and granulate with a 20-mesh sieve; dry the granules at 50°C, granulate with a 20-mesh sieve; add magnesium stearate , mixed and pressed into tablets, the weight of the tablet is 350±15mg, the hardness is 6±1kg, and it is made into 1000 tablets, that is to say.
Embodiment 3
[0034] Prescription: 4-[2-dimethylamino-1-(1-hydroxycyclohexyl)ethyl]phenyl 4-methylbenzoate hydrochloride 80g, HPMC K1500PH 105g, citric acid 17.5g, microcrystalline Cellulose 140.5g, 80% ethanol solution 180ml, sodium stearyl fumarate 7g
[0035]Preparation method: Weigh 4-[2-dimethylamino-1-(1-hydroxycyclohexyl)ethyl]phenyl hydrochloride, hydroxypropyl methylcellulose, Mix microcrystalline cellulose and citric acid evenly; add an appropriate amount of 80% ethanol solution to the above mixed powder to prepare a soft material, and granulate with a 20-mesh sieve; dry the granules at 50°C, and granulate with a 20-mesh sieve; Sodium maleate, mixed and pressed into tablets, tablet weight 350±15mg, hardness 6±1kg, made into 1000 tablets, ready to go.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com