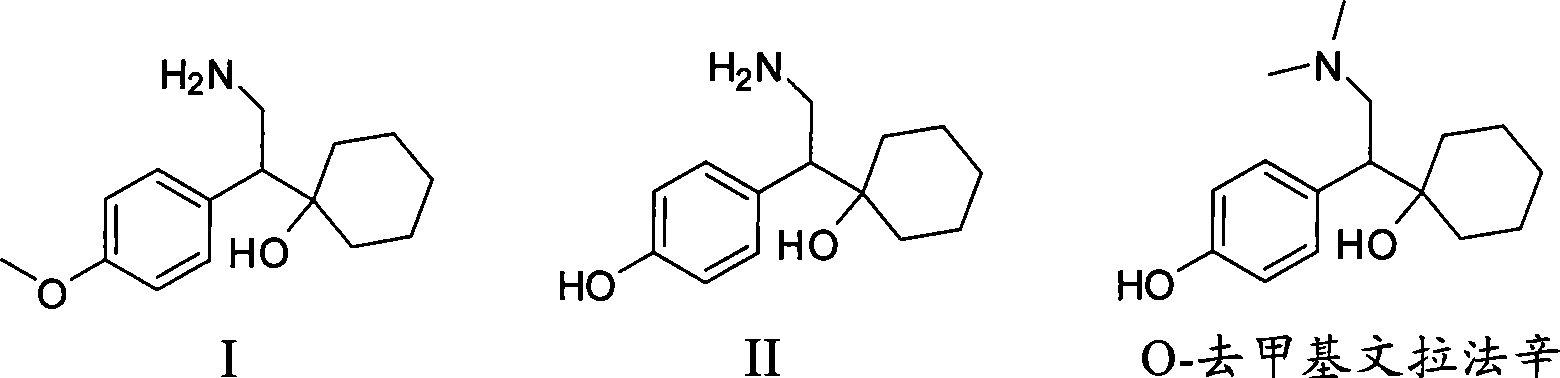
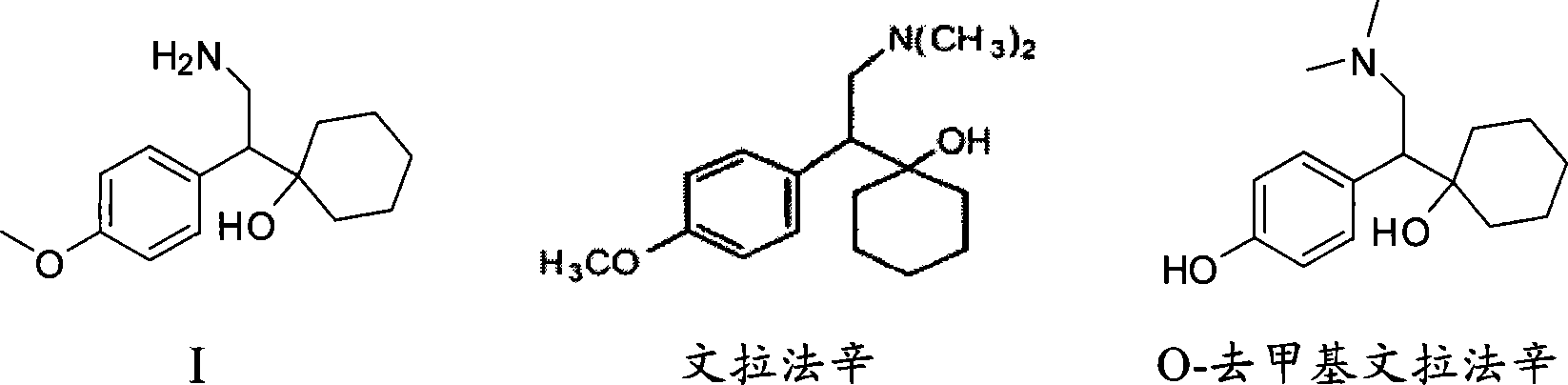
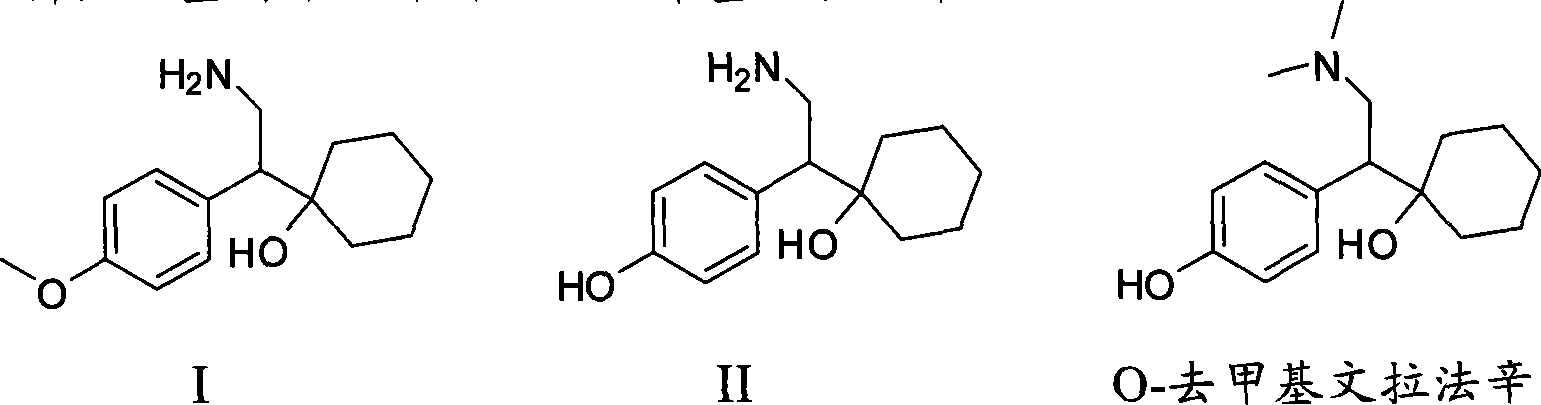
Novel method for preparing O-desvenlafaxine
A technology of desvenlafaxine and a new method, applied in the field of chemical pharmacy, can solve the problems of expensive sales, increased production cost and high production cost, and achieves the effects of simple operation, reduced production cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Compound I (1-[2-amino-1-(4-methoxyphenyl) ethyl] cyclohexanol) (Zhejiang Zhongbei Chemical Co., Ltd.) 50g, sodium methoxide (28%, Zibo Xusheng Chemical Co., Ltd.) 40g, dodecanethiol (Shanghai Nanxiang Reagent Co., Ltd.) 60g and PEG400 (molecular weight 380-430, Shanghai Qingxi Chemical Technology Co., Ltd.) React for 4 hours. Then cool to 80°C, add 100ml of water, then cool to normal temperature, let stand, separate layers, and filter the lower water layer. The aqueous phase was extracted four times with 100 ml of dichloromethane each time, and the dichloromethane was combined. Then, concentrated hydrochloric acid was added dropwise to the aqueous phase until the isoelectric point, pH = 9.5, and a large amount of solids were precipitated. Stir at room temperature for 30 minutes, then cool to 0-5°C and keep warm for 1 hour, filter to obtain a white solid, and dry to obtain intermediate compound II (1-[2-amino-1-(4-hydroxyphenyl)ethyl ] cyclohexanol) 38.1g. Purity (H...
Embodiment 2
[0027] Add 50g of compound I (1-[2-amino-1-(4-methoxyphenyl)ethyl]cyclohexanol), 40g of sodium methoxide, 60g of dodecanethiol and 133g of PEG400 into the reactor, stir and raise the temperature To 150°C, react at 150-155°C for 5 hours. Then cool to 80°C, add 100ml of water, then cool to normal temperature, let stand, separate layers, and filter the lower water layer. The aqueous phase was extracted four times with 100 ml of dichloromethane each time, and the dichloromethane was combined. Then, concentrated hydrochloric acid was added dropwise to the aqueous phase until the isoelectric point, pH = 10, and a large amount of solids were precipitated. Stir at room temperature for 30 minutes, then cool to 0-5°C and keep warm for 1 hour, filter to obtain a white solid, and dry to obtain intermediate compound II (1-[2-amino-1-(4-hydroxyphenyl)ethyl ] cyclohexanol) 31.3g. Purity (HPLC): 90.4%.
[0028] 31.3 g of the obtained compound II was added to 400 ml of dichloromethane, hea...
Embodiment 3
[0030] Add 50g of compound I (1-[2-amino-1-(4-methoxyphenyl)ethyl]cyclohexanol), 40g of sodium methoxide, 60g of dodecanethiol and 133g of PEG400 into the reactor, stir and raise the temperature To 210°C, react at 215-220°C for 2 hours. Then cool to 80°C, add 100ml of water, then cool to normal temperature, let stand, separate layers, and filter the lower water layer. The aqueous phase was extracted four times with 100 ml of dichloromethane each time, and the dichloromethane was combined. Then, concentrated hydrochloric acid was added dropwise to the aqueous phase until the isoelectric point, pH = 9.5, and a large amount of solids were precipitated. Stir at room temperature for 30 minutes, then cool to 0-5°C and keep warm for 1 hour, filter to obtain a white solid, and dry to obtain intermediate compound II (1-[2-amino-1-(4-hydroxyphenyl)ethyl ] cyclohexanol) 36.1g. Purity (HPLC): 96.2%.
[0031] 36.1 g of the obtained compound II was added to 400 ml of dichloromethane, he...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com