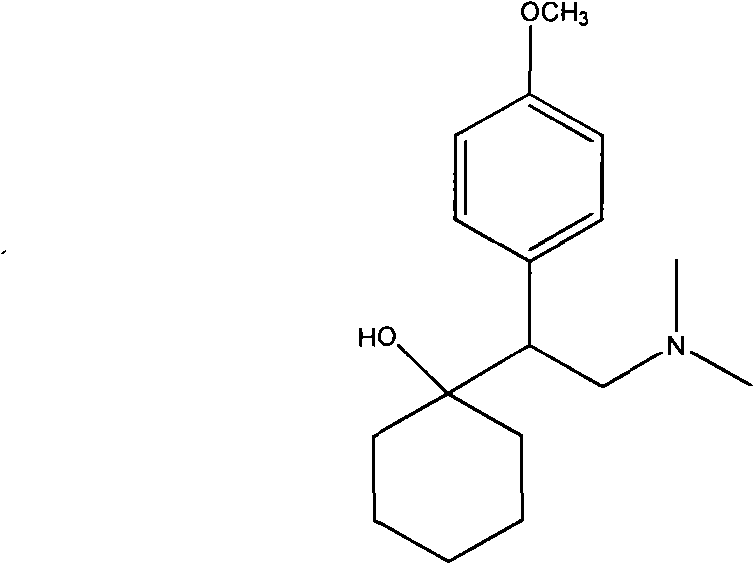
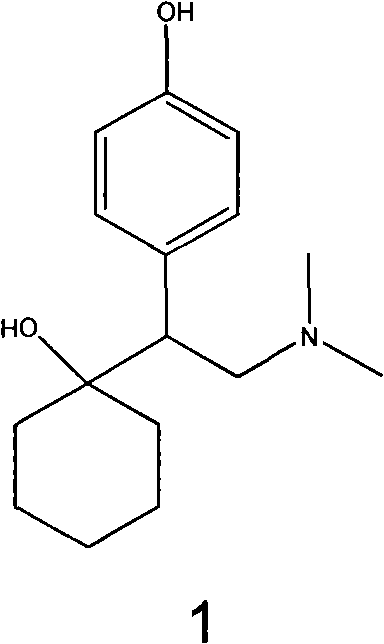
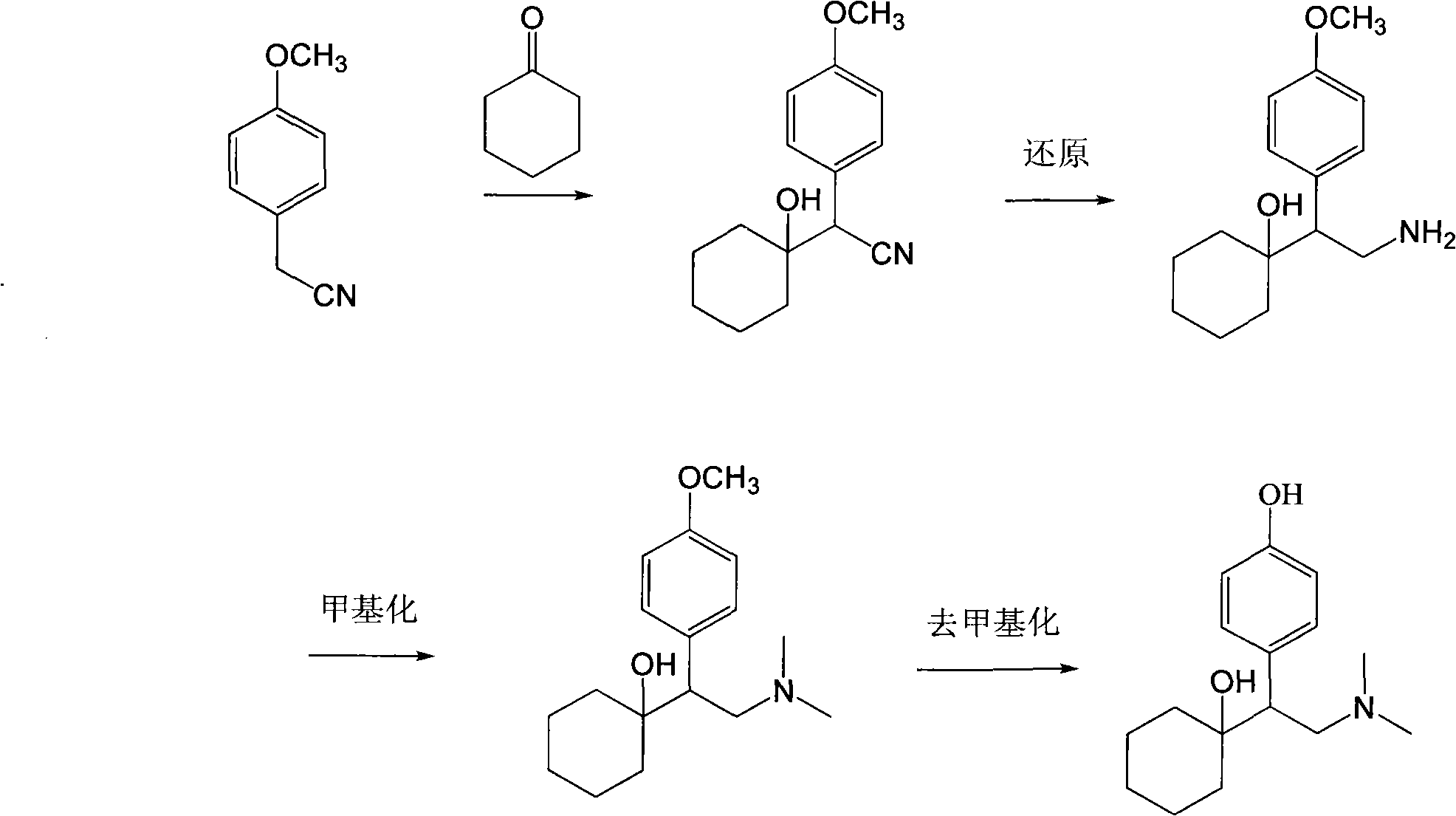
Preparation method of O-desmethylvenlafaxine
A technology of methyl ethylene and butyl lithium, which is applied in the preparation of organic compounds, preparation of amino hydroxyl compounds, chemical instruments and methods, etc., can solve the problem of difficult control of methylation reaction conditions, difficulty in obtaining highly toxic starting materials, cyanide Harsh base reduction conditions and other problems, to achieve the effect of mild reaction conditions, simplified reaction steps, and reduced pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0034] The preferred implementation of the present invention will be described in detail below according to the examples.
[0035] 1, 2-(4-hydroxyphenyl)-N, the preparation of N-dicarboxamide (3)
[0036] Add p-hydroxyphenylacetic acid 2 (10g) and dichloromethane (50ml) into a 100ml reaction flask, cool down to 0°C, and slowly add 10ml of thionyl chloride dropwise. After reacting for 2h, dichloromethane was distilled off to obtain acid chloride.
[0037] Add 50ml of dimethylamine aqueous solution into a 100ml reaction flask, add acid chloride dropwise, react for 1h, and use pure ethyl acetate thin-layer chromatography (TLC), R f After the thin layer showed that the reaction was complete, the product (3) was obtained by filtration, and dried to obtain 9.8 g of off-white powdery solid (purity 98%). 1 H NMR (DMSO-d6) δ: 9.22(1H), 7.01(2H), 6.71(2H), 3.54(2H), 2.96(3H), 2.81(3H).
[0038] 2. Preparation of 2-(1-hydroxycyclohexyl)-2-(4-hydroxyphenyl)-N,N-dicarboxamide (4)
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com