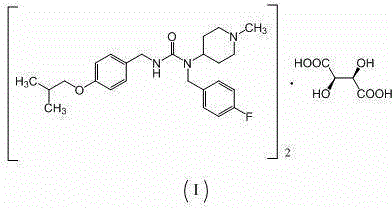
Preparation method of substituted urea derivative
An intermediate, isobutoxybenzyl technology, applied in the field of chemical pharmacy, can solve the problems of raw material toxicity, high price, side reactions, etc., and achieve the effects of high product purity and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1. Preparation of N-(4-fluorobenzyl)-1-methylpiperidin-4-amine hydrochloride (intermediate of formula III)
[0032] In a 10L dry reaction kettle, add 6000ml of isopropanol and N-(4-fluorobenzyl)-1-methylpiperidin-4-amine (Formula V) (555g, 2.5mol), stir, and cool to 0~ Add a hydrogen chloride solution of isopropanol dropwise at 5°C, adjust the pH to 1-2, keep stirring for 1 hour, filter, wash the solid with an appropriate amount of isopropanol, and dry it under vacuum at 70-80°C to obtain N-(4-fluorobenzyl)- 674g of 1-methylpiperidin-4-amine hydrochloride, yield 91.3%.
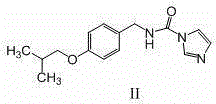
[0033] 2. N-(4-isobutoxybenzyl)-1 H - Preparation of imidazole-carboxamide (formula II intermediate)
[0034] In a 20L dry reaction kettle, at room temperature, add 2500ml of dry N,N'-dimethylformamide, 8500ml of dry acetonitrile, and add (4-isobutoxyphenyl)-methylamine (formula IV ) (538g, 3.0mol) and N,N'-carbonyldiimidazole (CDI) (584g, 3.6mol), stirred at 20-25°C for 15h, after the reaction was c...
Embodiment 2
[0046] 1. Preparation of N-(4-fluorobenzyl)-1-methylpiperidin-4-amine hydrochloride (intermediate of formula III)
[0047] In a 2000ml dry three-necked flask, add 600ml of isopropanol and N-(4-fluorobenzyl)-1-methylpiperidin-4-amine (Formula V) (55.5g, 0.25mol), stir and cool to 0 ~5°C, add isopropanol hydrogen chloride solution dropwise, adjust the pH to 1~2, keep stirring for 1 hour, filter, wash the solid with an appropriate amount of isopropanol, and dry under vacuum at 70~80°C to obtain N-(4-fluorobenzyl) -1-methylpiperidin-4-amine hydrochloride 68g, yield 91.4%.
[0048] 2. N-(4-isobutoxybenzyl)-1 H - Preparation of imidazole-carboxamide (formula II intermediate)
[0049] In a 2000ml dry three-necked flask, at room temperature, add 250ml of dry N,N'-dimethylacetamide, 850ml of dry acetonitrile, and add (4-isobutoxyphenyl)-methylamine (formula IV ) (53.8g, 0.3mol) and N,N'-carbonyldiimidazole (CDI) (58.4g, 0.36mol), stirred and reacted at 20-25℃ for 15h, after the reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com