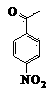
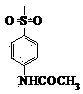
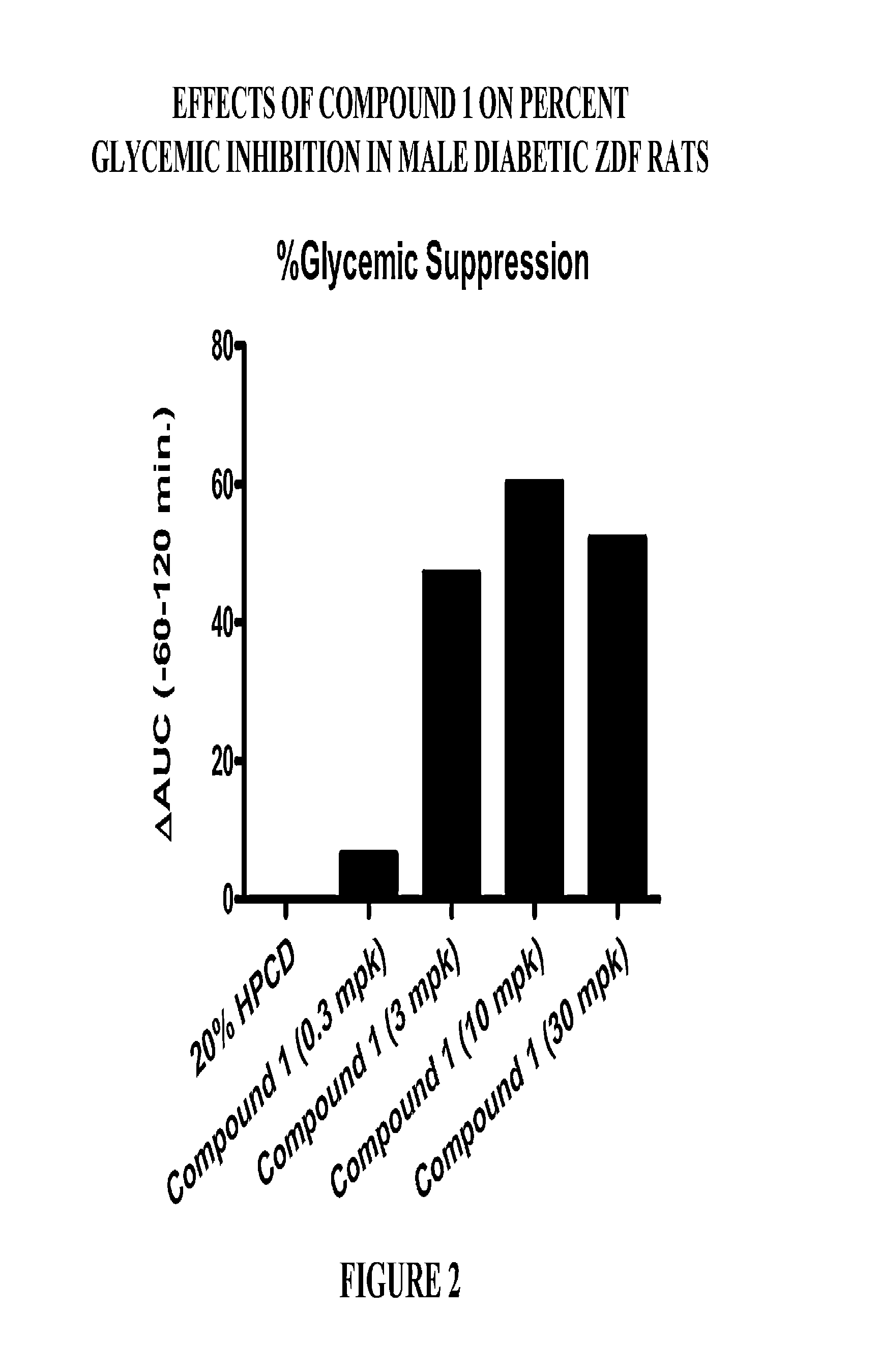
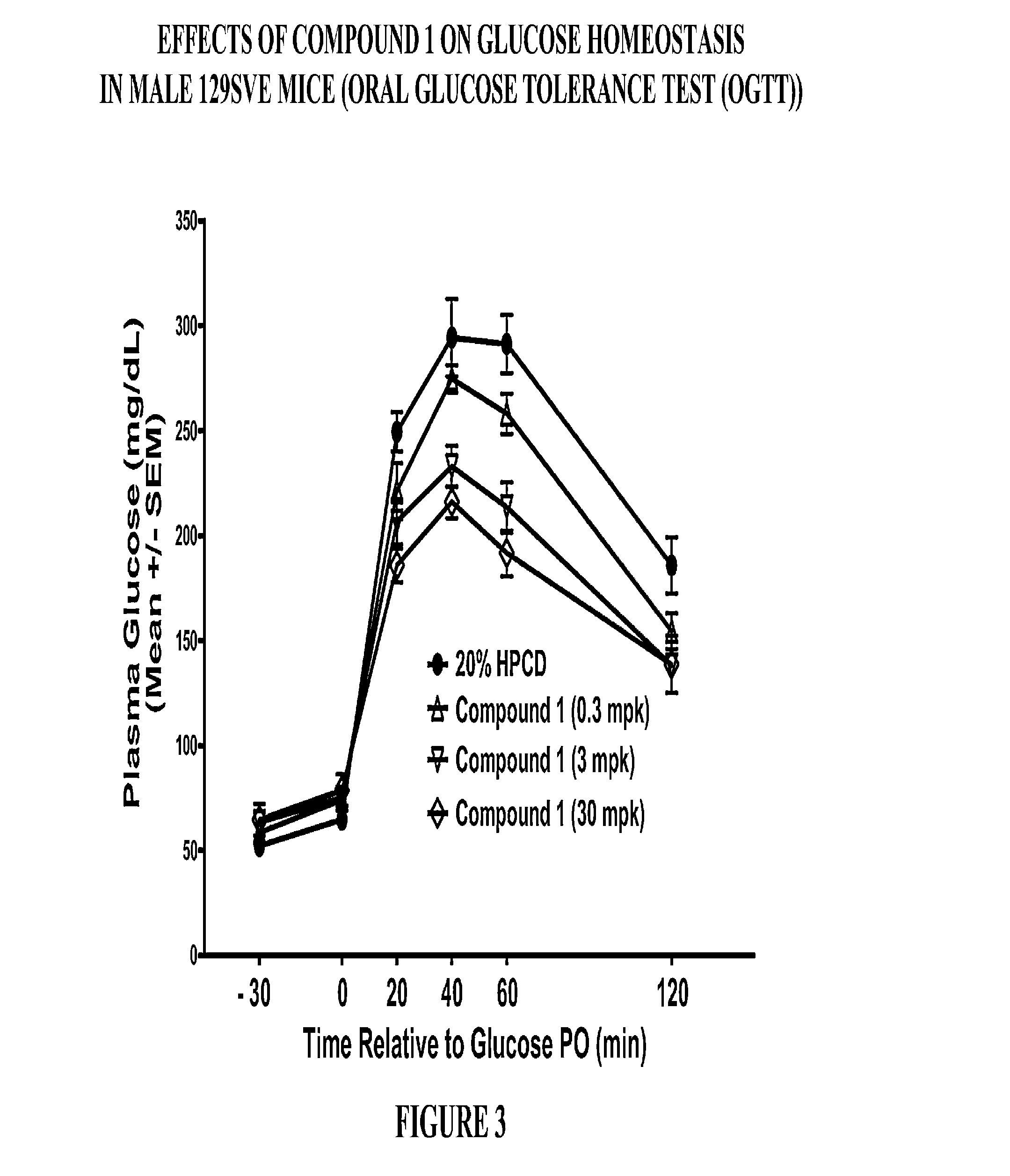
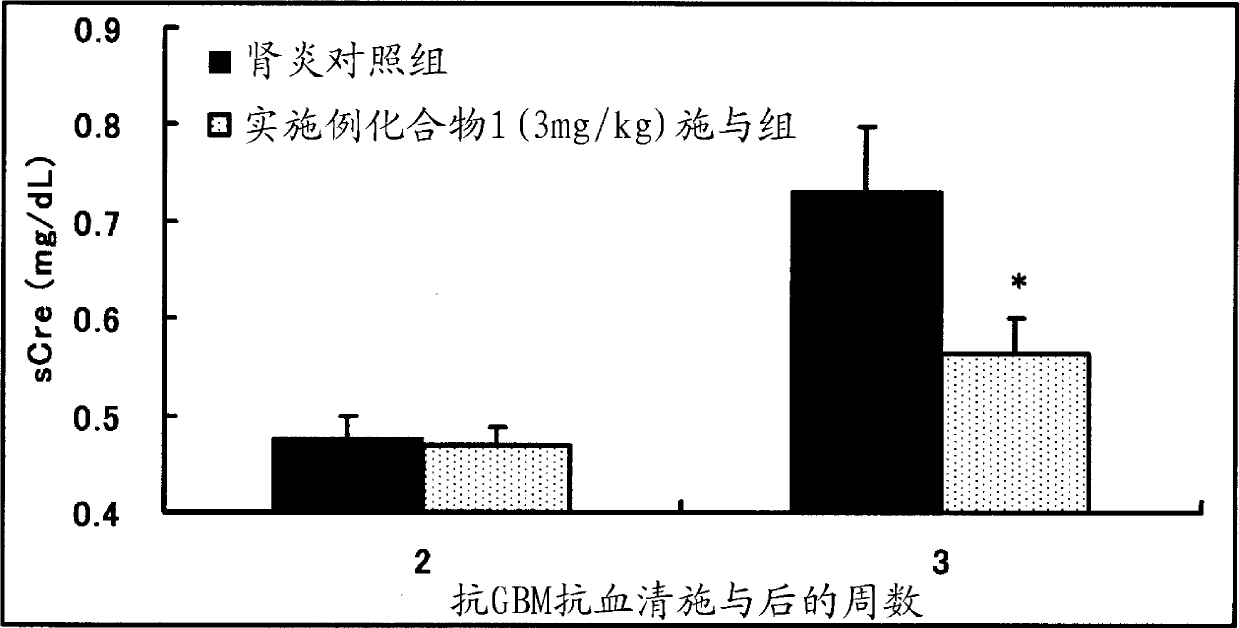
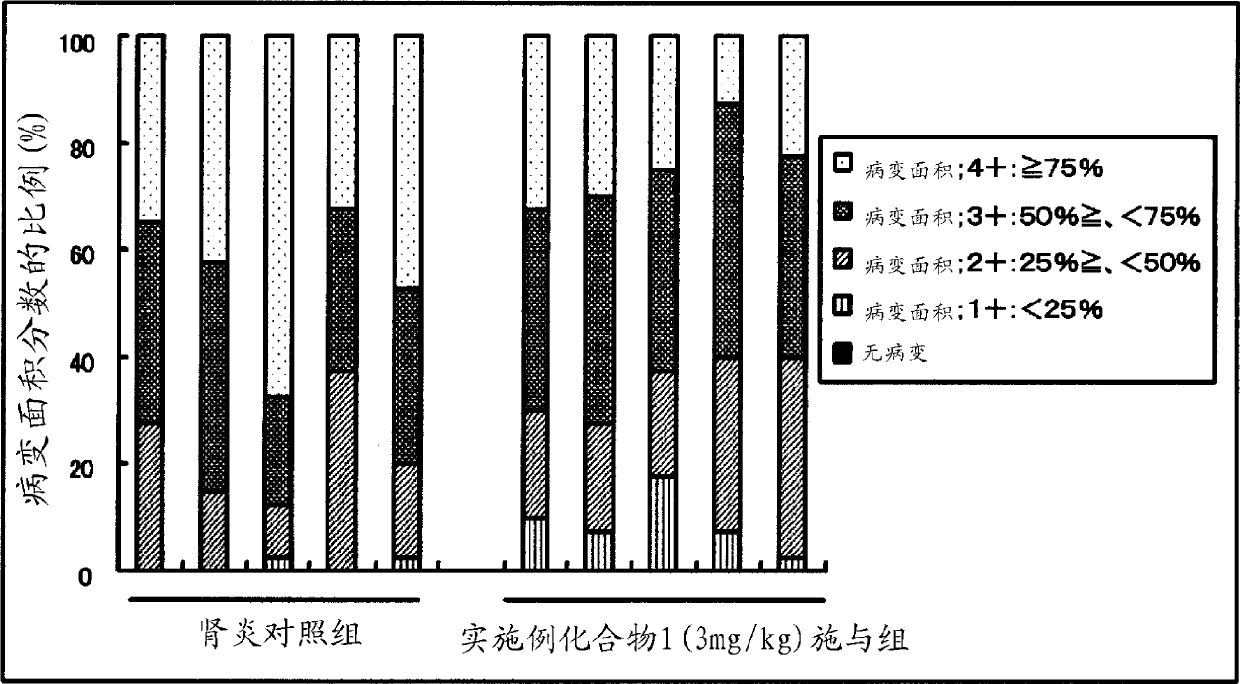
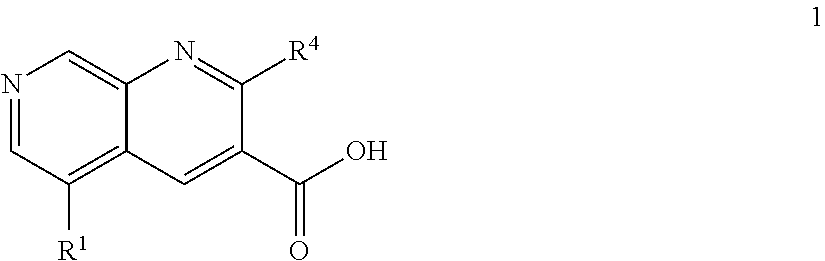
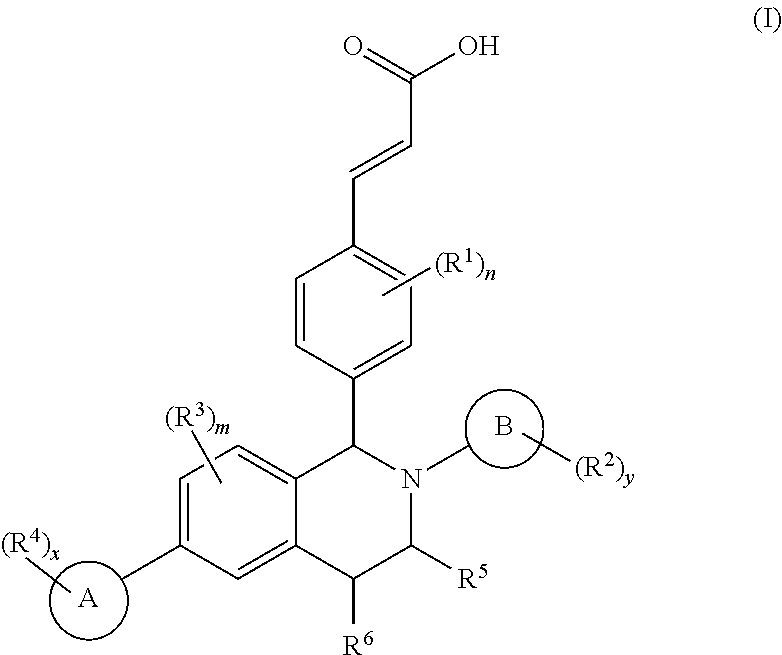
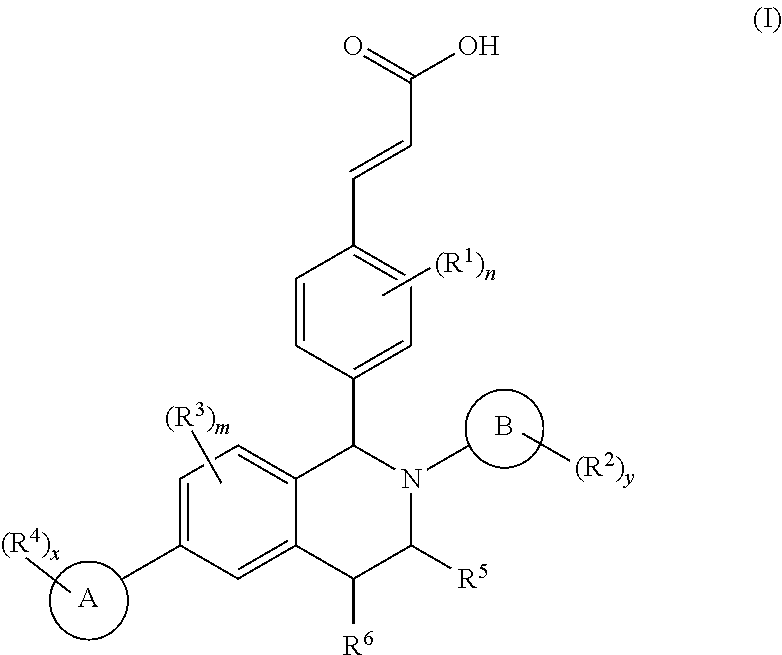
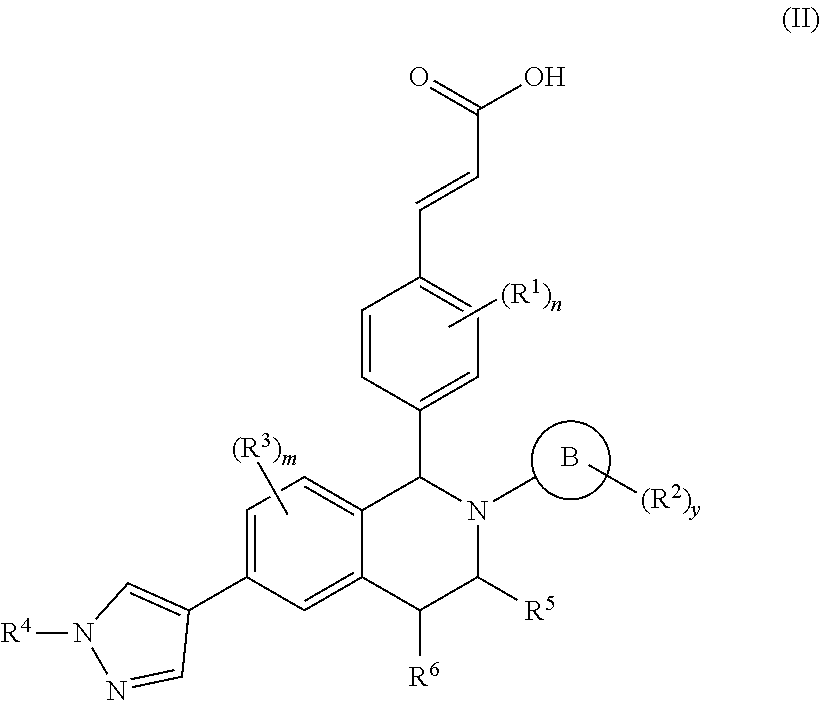
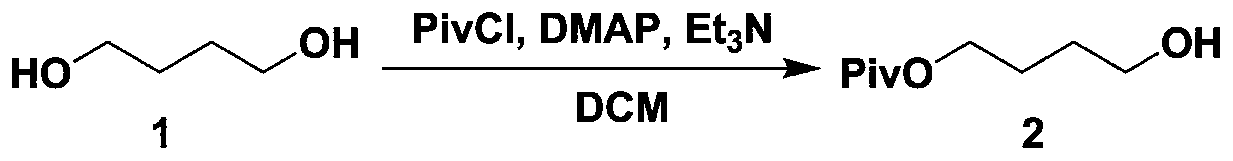
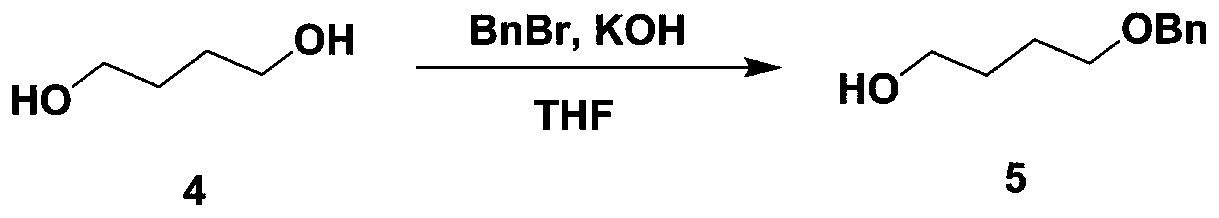
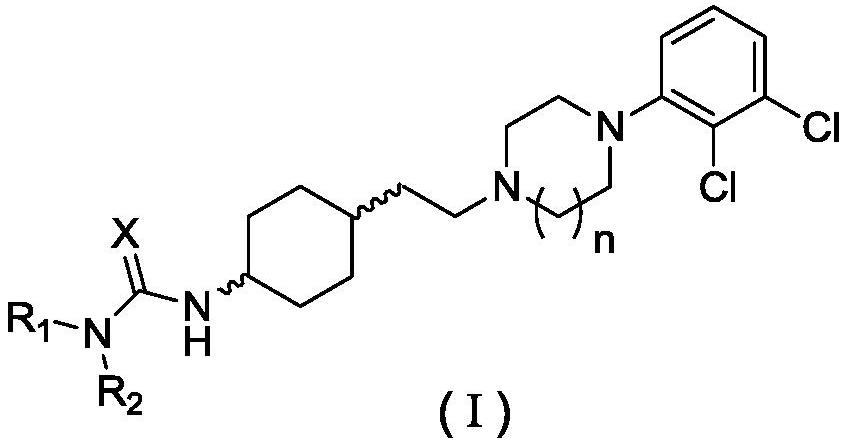
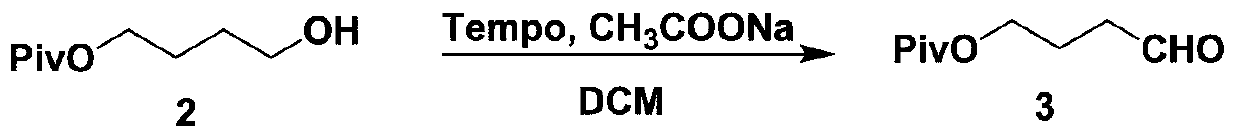Patents
Literature
38 results about "Pipequaline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pipequaline (INN) (developmental code name PK-8165) is an anxiolytic drug that was never marketed. It possesses a novel chemical structure that is not closely related to other drugs of this type. The drug has a similar pharmacological profile to the benzodiazepine family of drugs, but with mainly anxiolytic properties and very little sedative, amnestic or anticonvulsant effects, and so is classified as a nonbenzodiazepine anxiolytic.
Nicotinamide acids, amides, and their mimetics active as inhibitors of PDE4 isozymes
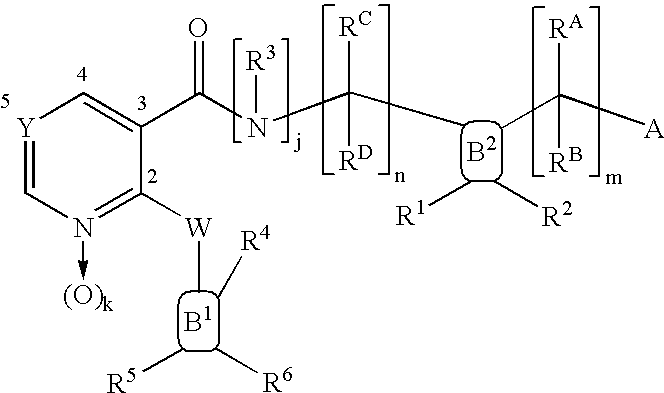
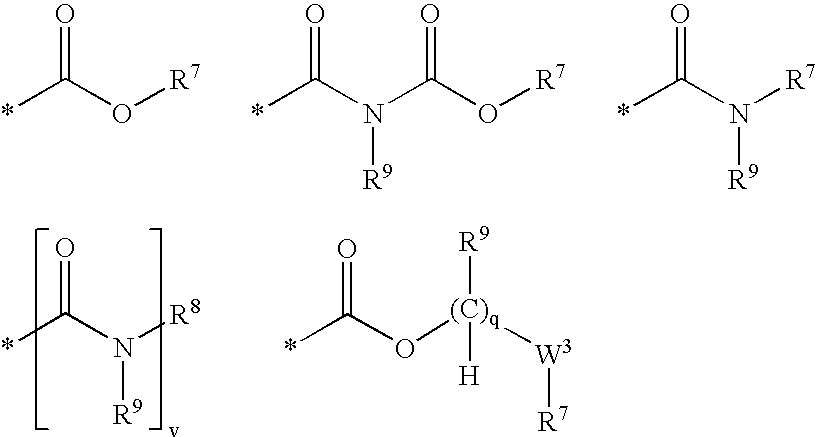
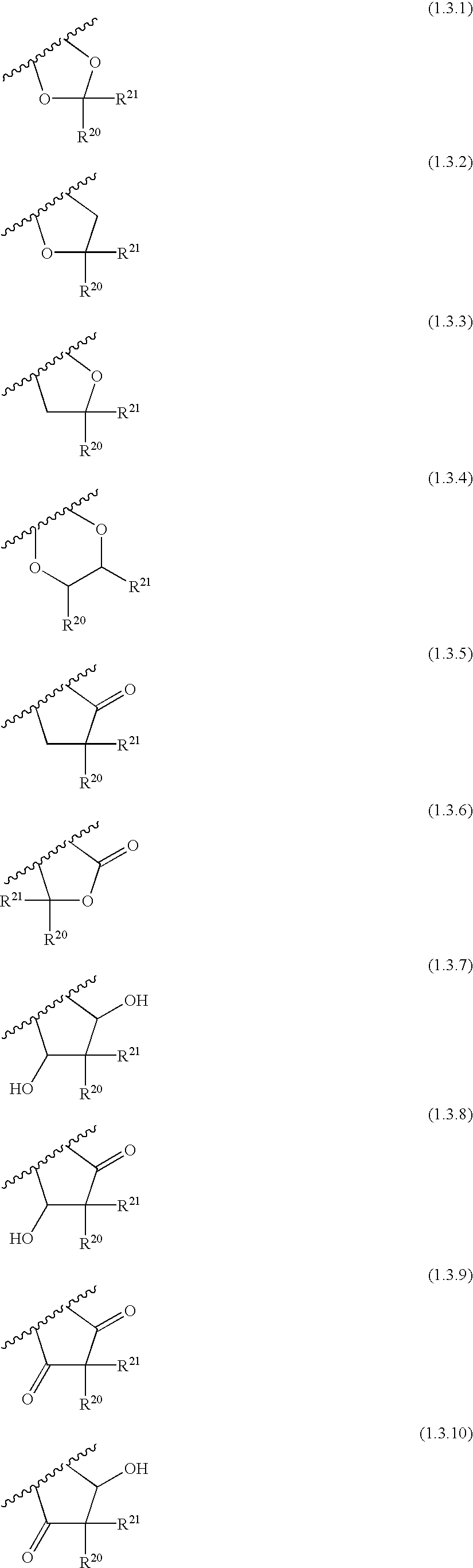
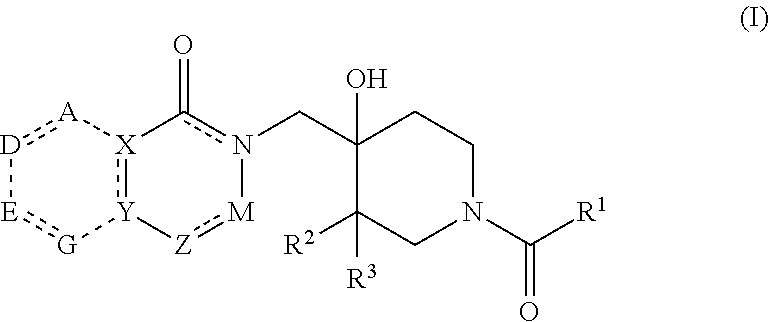
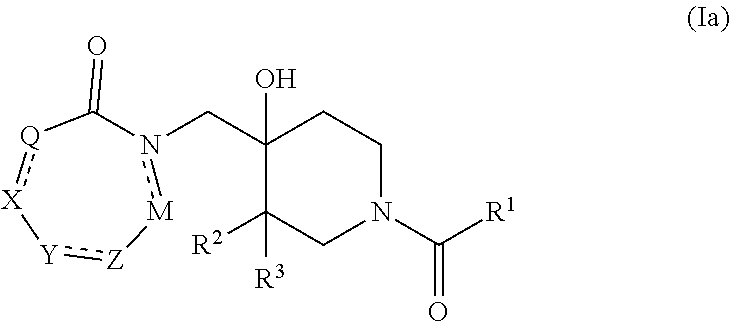
Compounds useful as inhibitors of PDE4 in the treatment of diseases regulated by the activation and degranulation of eosinophils, especially asthma, chronic bronchitis, and chronic obstructuive pulmonary disease, of the formula: wherein j is 0 or 1, k is 0 or 1, m is 0, 1, or 2; n is 1 or 2; A is selected from the partial Formulas: where q is 1, 2, or 3, W3 is -O-; -N(R9)-; or -OC(=O)-; R7 is selected from -H; -(C1-C6) alkyl, -(C2-C6) alkenyl, or -(C2-C6) alkynyl substituted by 0 to 3 substituents R10; -(CH2)u-(C3-C7) cycloalkyl where u is 0, 1 or 2, substituted by 0 to 3 R10; and phenyl or benzyl substituted by 0 to 3 R14; R8 is tetrazol-5-yl; 1,2,4-triazol-3-yl; 1,2,4-triazol-3-on-5-yl; 1,2,3-triazol-5-yl; imidazol-2-yl; imidazol-4-yl; imidazolidin-2-on-4-yl; 1,3,4-oxadiazolyl; 1,3,4-oxadiazol-2-on-5-yl; 1,2,4-oxadiazol-3-yl; 1,2,4-oxadiazol-5-on-3-yl; 1,2,4-oxadiazol-5-yl; 1,2,4-oxadiazol-3-on-5-yl; 1,2,5-thiadiazolyl; 1,3,4-thiadiazolyl; morpholinyl; parathiazinyl; oxazolyl; isoxazolyl; thiazolyl; isothiazolyl; pyrrolyl; pyrazolyl; succinimidyl; glutarimidyl; pyrrolidonyl; 2-piperidonyl; 2-pyridonyl; 4-pyridonyl; pyridazin-3-onyl; pyridyl; pyrimidinyl; pyrazinyl; pyridazinyl; indolyl; indolinyl; isoindolinyl; benzo[b]furanyl; 2,3-dihydrobenzofuranyl; 1,3-dihydroisobenzofuranyl; 2H-1-benzopyranyl; 2-H-chromenyl; chromanyl; benzothienyl; 1H-indazolyl; benzimidazolyl; benzoxazolyl; benzisoxazolyl; benzothiazolyl; benzotriazolyl; benzotriazinyl; phthalazinyl; 1,8-naphthyridinyl; quinolinyl; isoquinolinyl; quinazolinyl; quinoxalinyl; pyrazolo[3,4-d]pyrimidinyl; pyrimido[4,5-d]pyrimidinyl; imidazo[1,2-a]pyridinyl; pyridopyridinyl; pteridinyl; or 1H-purinyl; or A is selected from phosphorous and sulfur acid groups; W is -O-; -S(=O)t-, where t is 0, 1, or 2; or -N(R3)-; Y is =C(R1a)-, or -[N<custom-character file="US20020111495A1-20020815-P00900.TIF" wi="20" he="20" id="custom-character-00001" / >(O)k] where k is 0 or 1; R4, R5 and R6 are (1) -H; provided that R5 and R6 are not both -H at the same time, -F; -Cl; -(C2-C4) alkynyl; -R16; -OR16; -S(=O)pR16; -C(=O)R16, -C(=O)OR16, -C(=O)OR<highlight><sup
Owner:PFIZER INC
Preparation method of substituted urea derivative
InactiveCN105111135ANo pollution in the processSimple stepsOrganic chemistryUrea derivativesPimavanserin tartrate
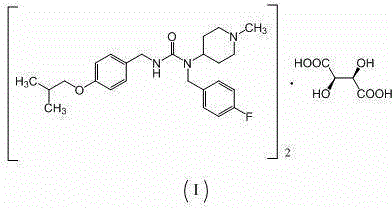
The invention relates to a novel preparation method of 1-(4-fluorobenzyl)-3-(4-isobutoxybenzyl)-1-(1-methylpiperidyl-4-yl)urea hemitartrate (pimavanserin tartrate), particularly a pharmaceutical compound for treating Parkinson's disease, of which the structural formula is disclosed as Formula (I). The invention also relates to a novel intermediate of the preparation method.
Owner:ANHUI YIXINMING PHARMA TECH
2-((2-(3-aminopiperidine-1)-4-oxythiophene (3, 2-d) pyrimidine-3(4H)-methyl) polymorphic benzonitrile, and preparation method and pharmacological applications thereof
The invention discloses 2-((2-(3-aminopiperidine-1)-4-oxythiophene (3, 2-d) pyrimidine-3(4H)-methyl) polymorphic benzonitrile, and a preparation method and pharmacological applications thereof. Compared with the prior art, the polymorphic benzonitrile has the advantages of high DPP4 (dipeptidyl peptidase-4) inhibitory activity, high in-vivo sugar reducing activity and fine pharmacokinetic characteristics, and is especially suitable for treatment of type 2 diabetes mellitus.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Antitumor 3,5-diphenylmethylene-4-piperidone derivative and preparation method thereof
The invention relates to an antitumor 3,5-diphenylmethylene-4-piperidone derivative and a preparation method thereof, and belongs to the technical fields of antitumor drugs and preparation methods thereof. The preparation method comprises the following steps: performing a Claisen-Schmidt condensation reaction on a 4-piperidone derivative having antitumor activity and 4-trifluoromethyl through 4-piperidone hydrochloride to obtain an intermediate a; and reacting the intermediate a with an acylation or sulfonylation reagent to obtain N-R-3,5-di(4-trifluoromethyl benzylidene)-4-piperidone b-5. The 3,5-diphenylmethylene-4-piperidone derivative is novel in structure, has the advantage of combination of a plurality of molecular targets, and has multidrug resistance activity; and the genotoxicity of currently-used antitumor drugs can be avoided. Compared with normal cells, the derivative has higher antitumor activity to tumor cells, so that the derivative has a great application prospect in the field of antitumor drugs.
Owner:BINZHOU MEDICAL COLLEGE
Therapeutic agent for breast cancer
PendingUS20180303817A1Reduce tumor volumeOrganic active ingredientsAntineoplastic agentsMethyl-1H-indoleMethyl group
The present application discloses a therapeutic agent for breast cancer, comprising 5-((2-(4-(1-(2-hydroxyethyl)piperidin-4-yl)benzamide)pyridin-4-yl)oxy)-6-(2-methoxyethoxy)-N-methyl-1H-indole-1-carboxamide or a pharmacologically acceptable salt thereof.
Owner:EISIA R&D MANAGEMENT CO LTD
Compound preparation for treating and preventing thrombosis and apoplexia as well as its preparing method
InactiveCN1480144AReduce manufacturing costSmall fluctuations in performanceSalicyclic acid active ingredientsBlood disorderBenzoic acidDipyridamole
A slowly-releasing compound medicine in the form of tablet for treating and preventing thrombosis and apoplexy is prepared from aspirin, 2-(acetoxy) benzoic acid, slow-releasing dipyridamole, 2,2',2'',2''', [(4,8-dipiperidylpyrimido[5,4-d] pyrimidine-2,6-diyl) dihyponitido]-tetraethanol through granulating, tabletting and coating. Its advantages are high curative effect and equickly taking its effect.
Owner:CHENGDU LIST PHARMA
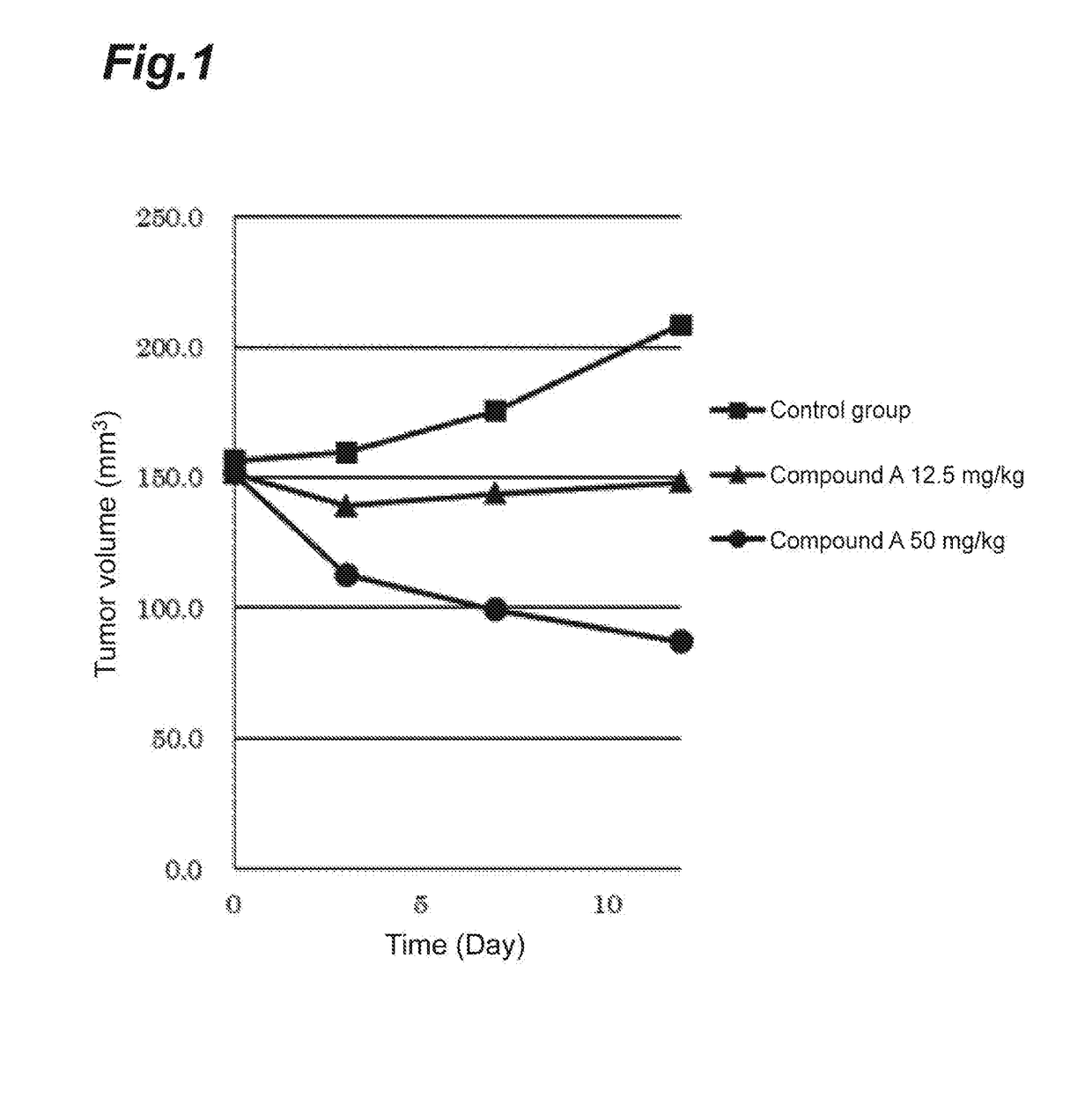
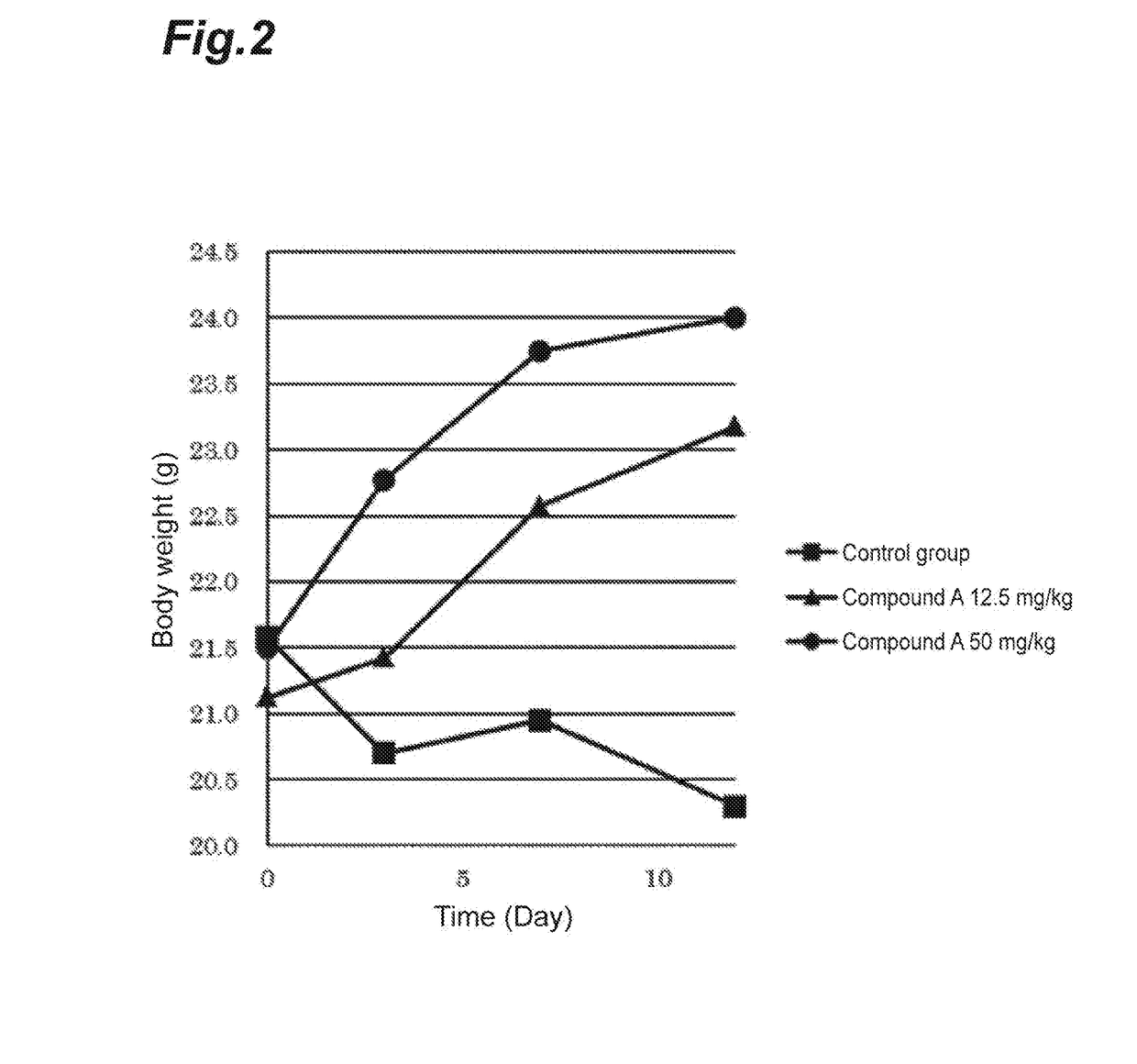
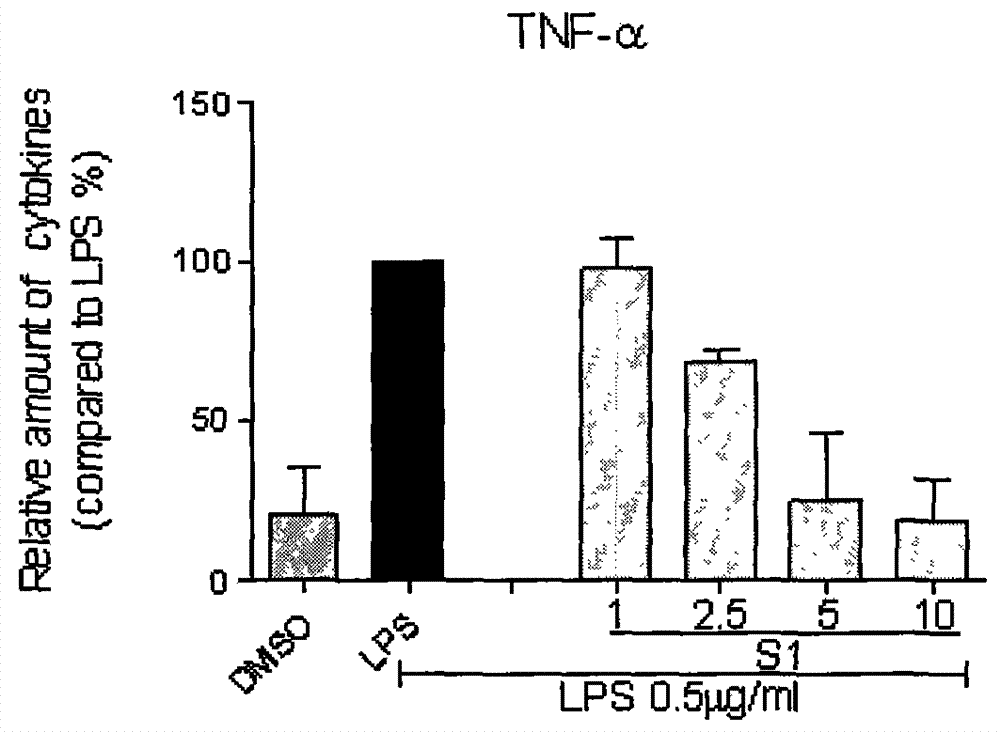
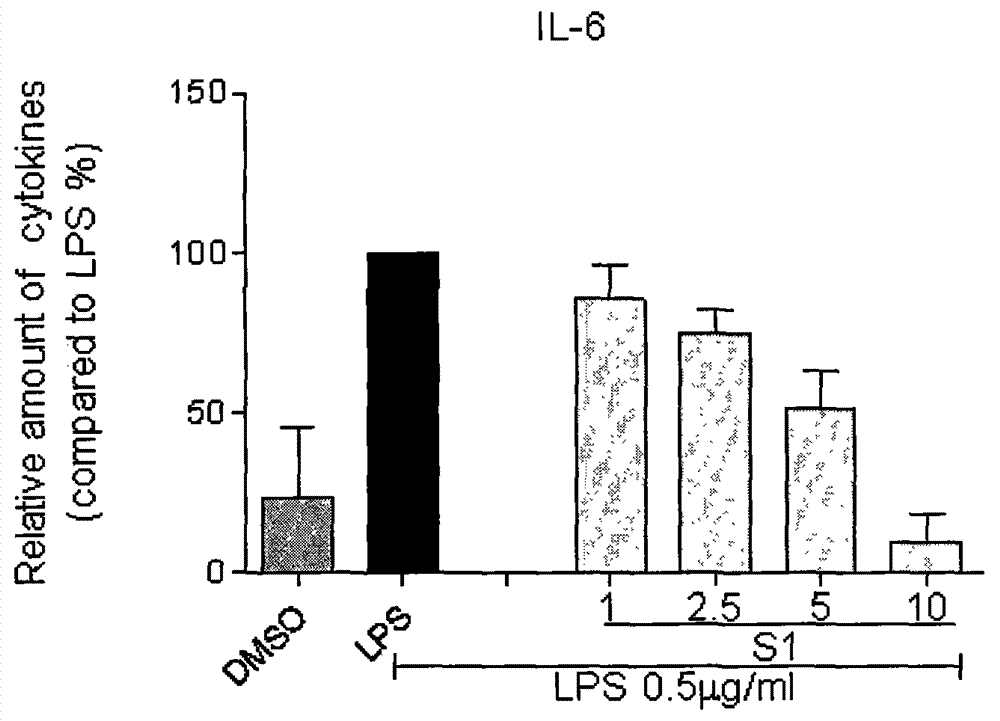
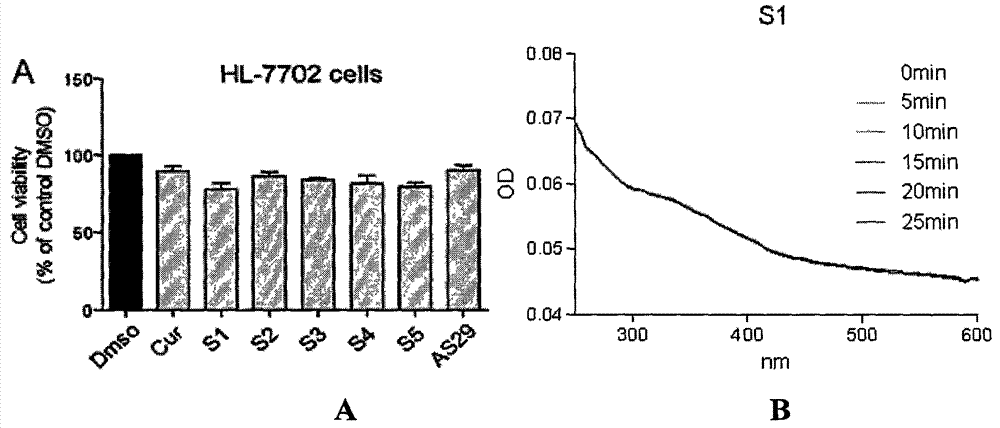
Application of curcumin analog S1 containing piperidone structure in preparation of anti-inflammation drugs
The invention belongs to the field of the pharmaceutical chemistry and pharmacology, and concretely relates to an application of a compound of [(1E,1'E)-(4-Oxo-1-propionylpiperidine-3,5-diylidene)bis(methanylylidene)]bis(2-methoxy-4,1-phenylene)dipropionate (S1) in the preparation of anti-inflammation or / and anti-oxidation drugs. The above piperidone type curcumin analog S1 can dose-dependently inhibit the expression and release of inflammation factors comprising TNF-alpha, IL-6 and the like, and can also obviously improve the survival rate of mice death-induced by LPS. The compound has a good safety and stability, and can be exploited to inflammation or / and oxidation stress related disease treatment drugs.
Owner:WENZHOU MEDICAL UNIV
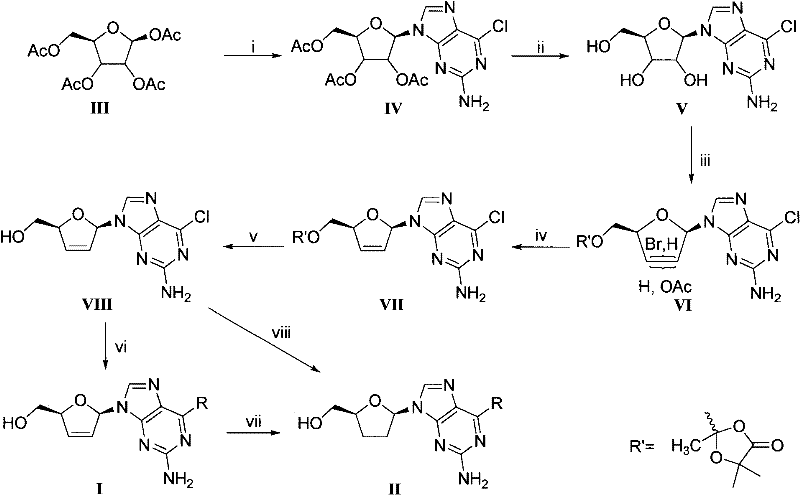
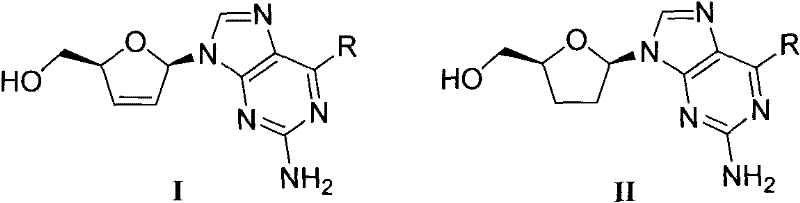
D, L-guanosine analogs, preparation methods thereof and applications thereof
The invention discloses D, L-guanosine analogs, preparation methods thereof and applications thereof. The structural formulas of the analogs are respectively represented by a general formula I or a general formula II, wherein R is hydrogen, amino, piperidyl, morpholinyl or pyrrolidinyl. The good chemical stability and the pharmacokinetic stability showed by the D, L-guanosine analogs of the invention make up for shortages of low stability, poor pharmacokinetic stability and the like existing in D4G and ddG. The preparation methods of the D, L-guanosine analogs have the advantages of mild reaction, simplicity and high yield. Antiviral activity testing shows that the compounds with the general formula I or the general formula II which have excellent antiviral activity can be applied to prepare anti-HIV drugs, anti-HBV drugs, anti-HCV drugs or anti-HSV drugs.
Owner:PEKING UNIV
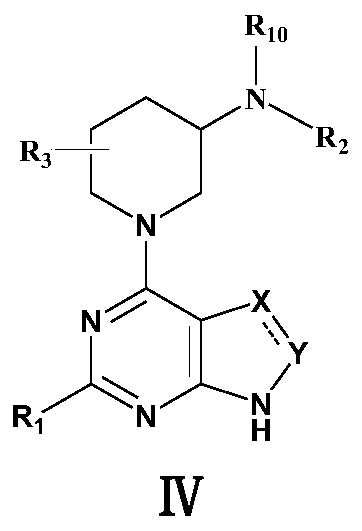
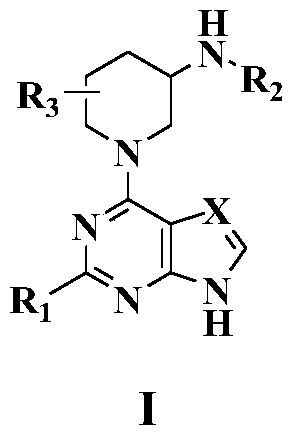
1-(pyrimidine-4-yl)-3-aminopiperdine derivative and its preparation method and use
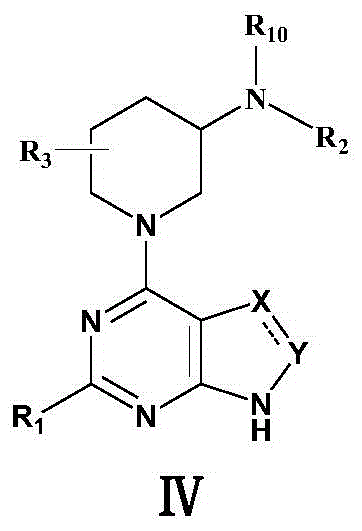
The invention relates to the field of chemical medicines and concretely relates to a 1-(pyrimidine-4-yl)-3-aminopiperdine derivative and its preparation method and use. The invention provides the 1-(pyrimidine-4-yl)-3-aminopiperdine derivative. The 1-(pyrimidine-4-yl)-3-aminopiperdine derivative has a structure shown in the formula IV. The invention also provides a preparation method and a use of the 1-(pyrimidine-4-yl)-3-aminopiperdine derivative. The 1-(pyrimidine-4-yl)-3-aminopiperdine derivative can selectively inhibit JAK3 and provide a novel choice for treating JAK3-related diseases such as rheumatoid arthritis, asthma, chronic obstructive pulmonary diseases and tumors.
Owner:CHENGDU ZENITAR BIOMEDICAL TECH CO LTD
Modulators of the gpr119 receptor and the treatment of disorders related thereto
The present invention relates to the GPR119 receptor agonists: 3-fluoro-4-(5-fluoro-6-(4-(3-(2-fluoropropan-2-yl)-1,2,4-oxadiazol-5-yl)piperidin-1-yl)pyrimidin-4-ylamino)-N,N-dimethylbenzamide; -fluoro-4-(5-fluoro-6-(4-(3-(2-fluoro-propan-2-yl)-1,2,4-oxadiazol-5-yl)piperidin-1-yl)pyrimidin-4-ylamino)-N-methylbenzamide; and 3-fluoro-4-(5-fluoro-6-(4-(3-(2-fluoropropan-2-yl)-1,2,4-oxadiazol-5-yl)piperidin-1-yl)pyrimidin-4-ylamino)benzamide, and pharmaceutically acceptable salts, solvates, and hydrates thereof, that are useful as a single pharmaceutical agent or in combination with one or more additional pharmaceutical agents, such as, a DPP-IV inhibitor, a biguanide, an alpha-glucosidase inhibitor, an insulin analogue, a sulfonylurea, an SGLT2 inhibitor, a meglitinide, a thiazolidinedione, or an anti-diabetic peptide analogue, in the treatment of for example, a disorder selected from: a GPR119-receptor-related disorder; a condition ameliorated by increasing secretion of an incretin; a condition ameliorated by increasing a blood incretin level; a condition characterized by low bone mass; a neurological disorder; a metabolic-related disorder; type 2 diabetes; obesity; and complications related thereto.
Owner:ARENA PHARMA
Nipecotic acid derivative and use thereof for medical purposes
InactiveCN104185626AGood treatment effectGood prevention effectOrganic chemistryUrinary disorderTreatment effectMedicine
The purpose of the present invention is to provide: a compound having an sEH-inhibiting activity; and a medicinal agent capable of exhibiting a therapeutic or prophylactic effect on chronic kidney diseases and pulmonary hypertension which relies on a sEH-inhibiting activity. The present invention provides a nipecotic acid derivative represented by chemical formula (1) or a pharmacologically acceptable salt thereof.
Owner:TORAY IND INC
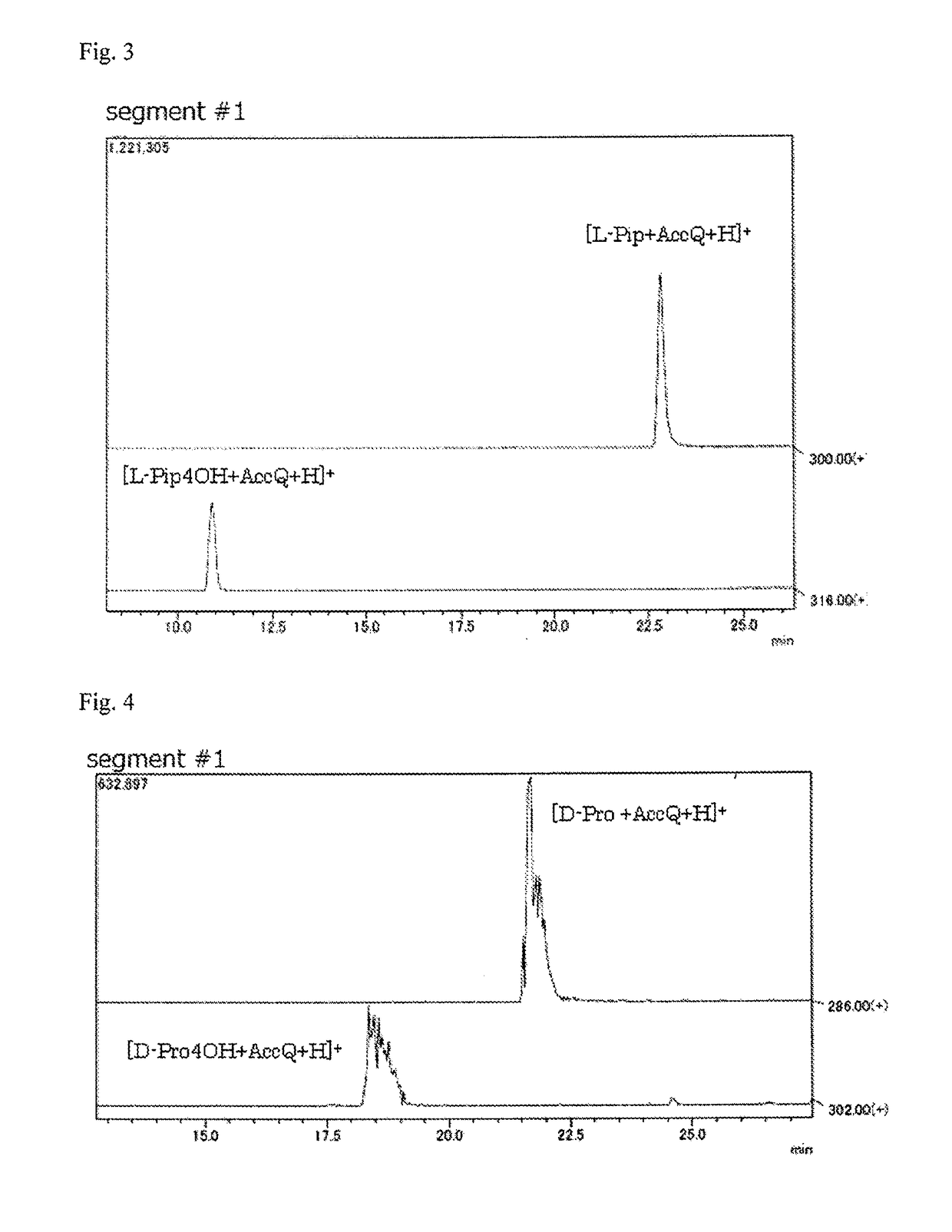
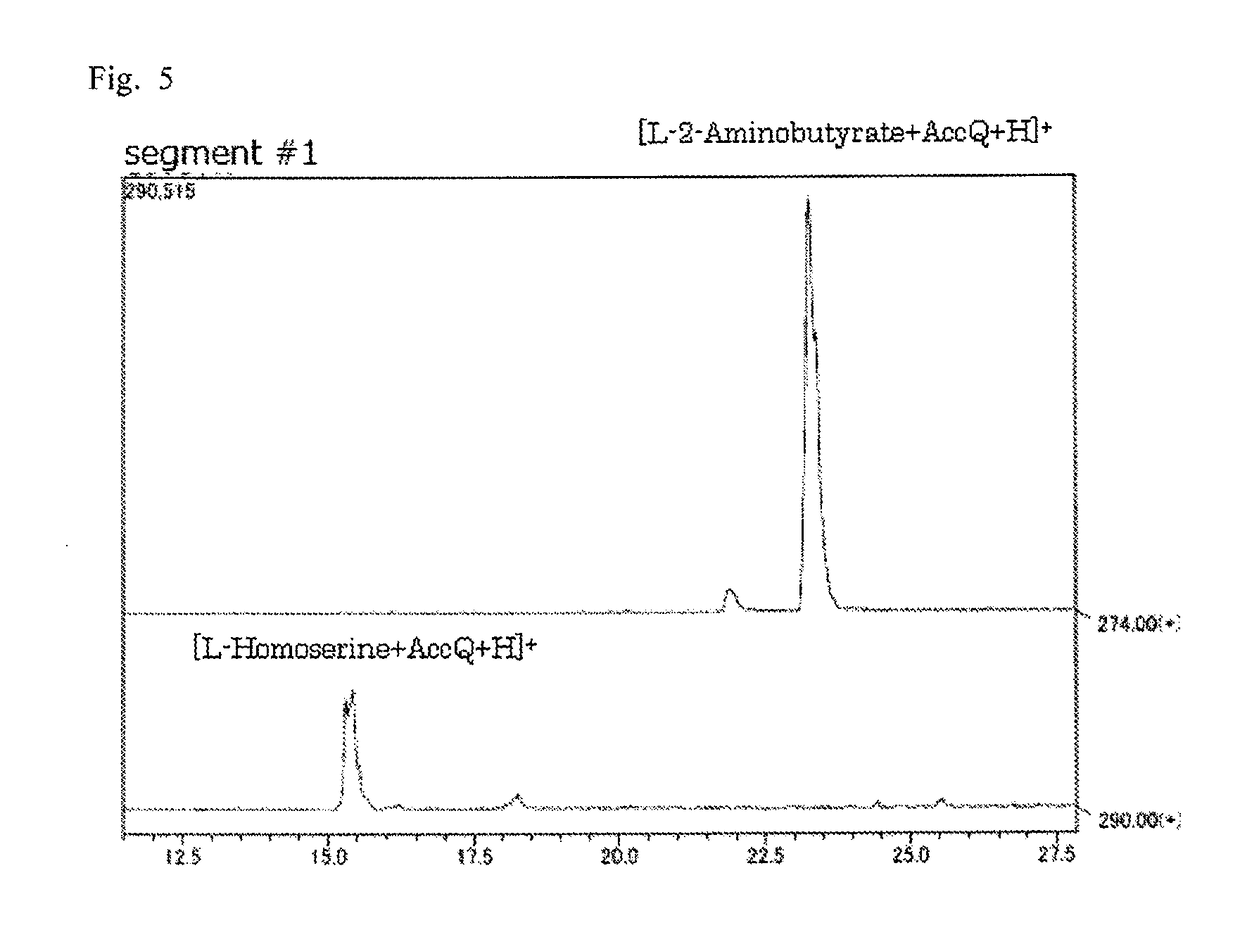
Pipecolinic acid 4-hydroxylase and method for producing 4-hydroxy amino acid using same
ActiveUS20160348081A1Efficient productionHigh optical purityBacteriaOxidoreductasesMugineic acidPipecolic acid
The present invention provides a pipecolic acid 4-hydroxylase protein exemplified by the following (A), (B), and (C), having activity to react with L-pipecolic acid in the presence of 2-oxoglutaric acid and iron(II) ions to produce trans-4-hydroxy-L-pipecolic acid, and a method for producing 4-hydroxy amino acid, which method comprises reacting the pipecolic acid 4-hydroxylase protein, cells containing the protein, a treated product of the cells, and / or a culture liquid obtained by culturing the cells, with α-amino acid to produce 4-hydroxy amino acid:(A) a polypeptide comprising the amino acid sequence represented by SEQ ID NO:2, 4, 6, 8, 10, 12, 16, or 18;(B) a polypeptide comprising the amino acid sequence represented by SEQ ID NO:2, 4, 6, 8, 10, 12, 16, or 18 except that one or several amino acids are deleted, substituted, and / or added, and having pipecolic acid 4-hydroxylase activity; and(C) a polypeptide having an amino acid sequence that is not less than 80% identical to the amino acid sequence represented by SEQ ID NO:2, 4, 6, 8, 10, 12, 16, or 18, and having pipecolic acid 4-hydroxylase activity.
Owner:API CORP (JP)
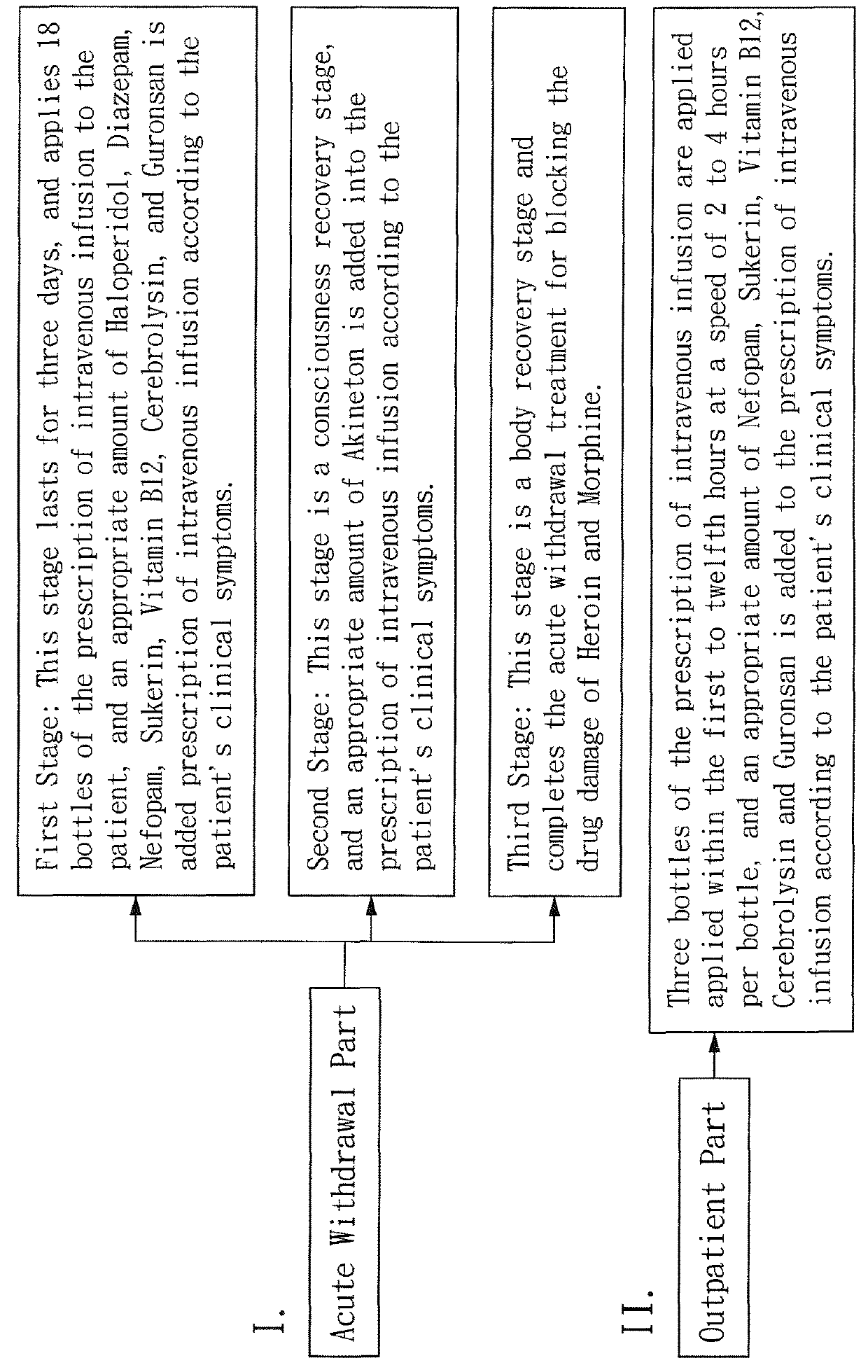
Prescription of intravenous medication for blocking heroin or morphine intoxication path and using thereof
ActiveUS10004781B2Enhancing withdrawalPrevent addictionNervous disorderPeptide/protein ingredientsMorphine poisoningBlock drugs
Owner:CHENG EN CHE
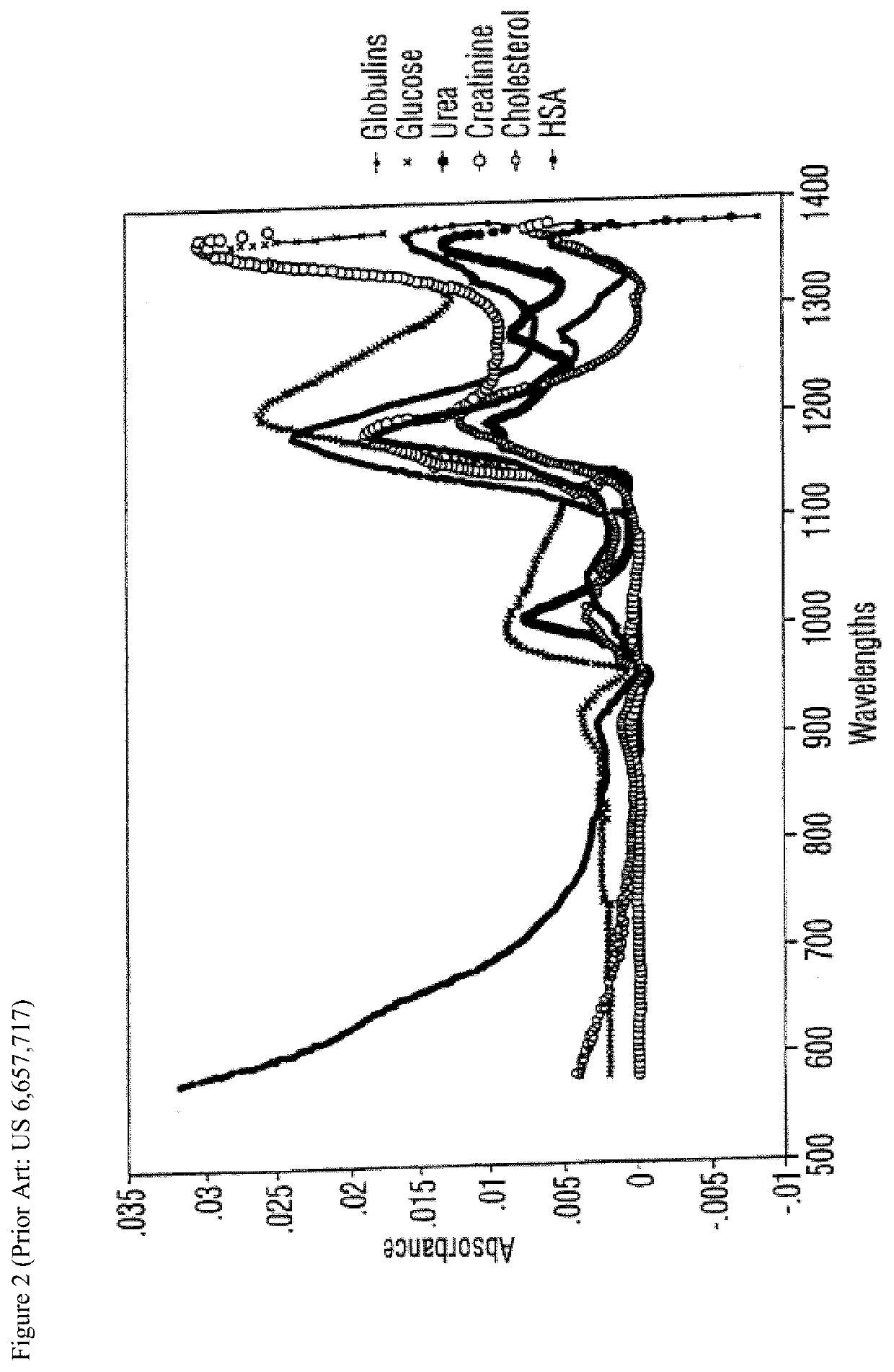
Non-invasive determination of a physiological state of interest in a subject
A method for non-invasively determining a physiological state of interest in a subject is described. The method involves, placing a body part in contact with a receptor, and directing a source of electromagnetic radiation (EMR) over a range of wavelengths onto body part so that the EMR reaches the blood and interstitial fluid within the body part. The EMR that is absorbed by, reflected by, or transmitted through, the blood and interstitial fluid of the body part is measured, and a spectrum over the range of wavelengths obtained. The spectrum is analyzed to determine an amount of two or more than two analytes within the blood and interstitial fluid of the body part and a biochemical profile is derived. The biochemical profile is used to determine the physiological state of interest in the subject. The physiological state of interest in the subject may be selected from the group of intoxication arising from cannabis, alcohol, a combination of cannabis and alcohol, opiates, fentanyl, amphetamines, phencyclidine, sedatives, anxyolytics, cocaine, caffeine, and nicotine consumption.
Owner:ISBRG CORP
Monocarbonyl curcumin analogue as well as preparation and application thereof
ActiveCN113354577AAdvance researchGood pharmacokinetic behaviorOrganic active ingredientsNervous disorderDiseasePipequaline
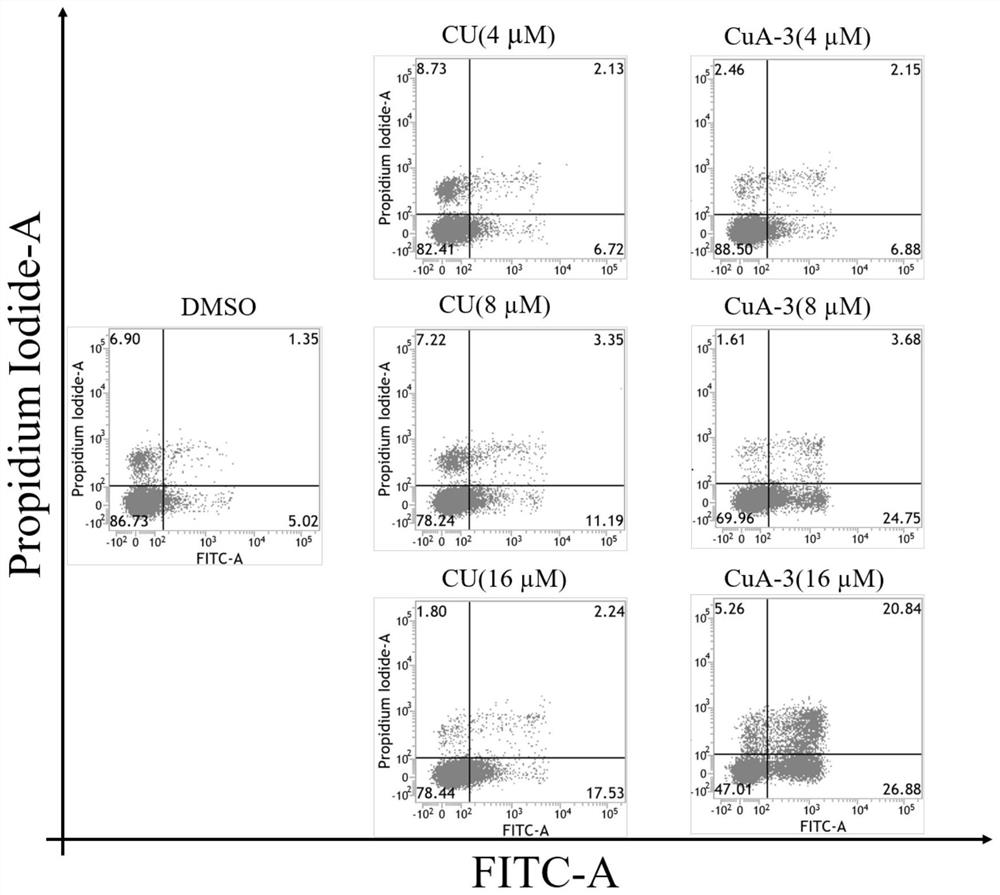
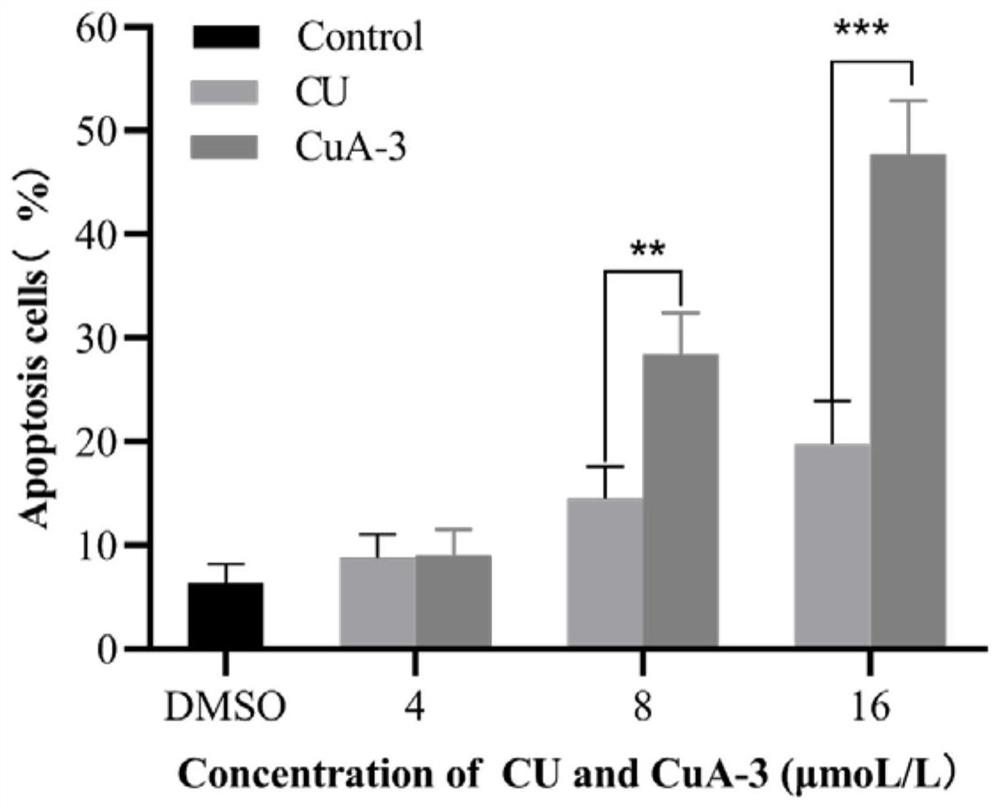
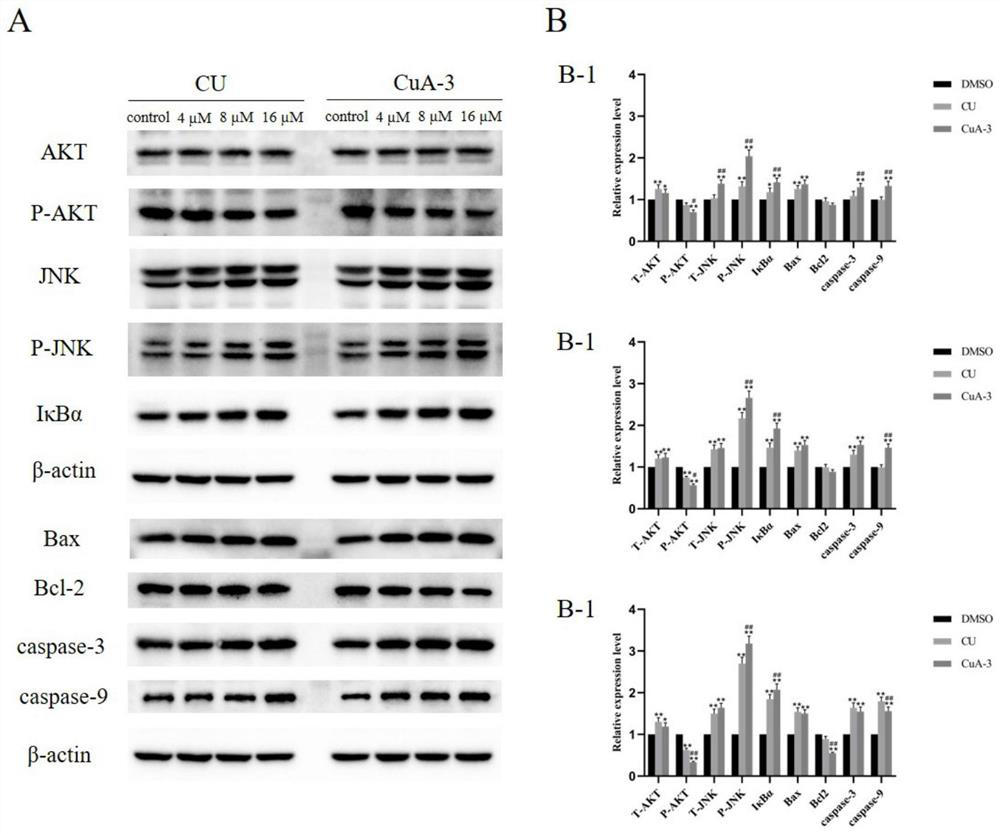
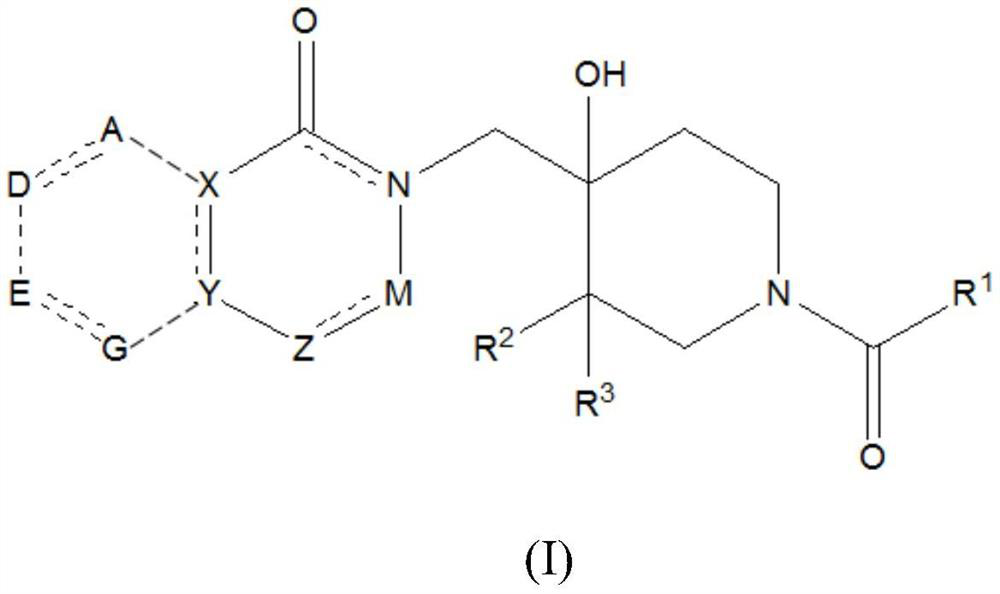
The invention discloses a monocarbonyl curcumin analogue as well as preparation and an application thereof, the curcumin analogue is represented by a formula I, or pharmaceutically acceptable salts of the curcumin analogue comprise hydrochloride and sulfate, aldehydes and ketones are used as raw materials, monocarbonyl curcumin analogues CuA-1-CuA-3 are designed and synthesized, by designing and synthesizing a monocarbonyl curcumin analogue CuA-4 by taking 3, 4-dihydro-2H-pyran, vanillin, 4-pyridinium methylbenzenesulfonate and 1-methyl-4-piperidone as raw materials, and substituting an unstable beta-dicarbonyl structure with a monocarbonyl group to obtain the more stable monocarbonyl curcumin analogue CuA-1-CuA-4. The monocarbonyl curcumin has better pharmacokinetic behavior and higher anti-tumor activity, and the monocarbonyl curcumin is applied to preparation of drugs for resisting inflammation and treating inflammation-related diseases, Alzheimer's disease, Parkinson's disease, depression, lung cancer, liver cancer, breast cancer, colon cancer and cervical cancer.
Owner:THE AFFILIATED HOSPITAL OF TRADITIONAL CHINESE MEDICAL TO SOUTHWEST MEDICAL UNIV +1
4-hydroxypiperidine derivatives and their use as inhibitors of ubiquitin specific protease 19 (USP19)
Inhibitors of ubiquitin specific protease 19 (USP19) of Formula (I) are provided, together with pharmaceutical compositions comprising said inhibitors, and methods of use thereof. The compounds can beused in in the treatment of muscular atrophy, obesity, insulin resistance or type II diabetes or in reducing the loss of muscle mass.
Owner:ALMAC DISCOVERY LIMITED
1,7-naphthyridine derivatives
The present invention relates to compounds of general formula I wherein R1, R2, R3 and R4 are as defined herein which maybe used for the treatment of schizophrenia, obsessive-compulsivepersonality disorder, major depression, bipolar disorders, anxiety disorders, normal aging, epilepsy, retinal degeneration, traumatic brain injury, spinal cord injury, post-traumatic stress disorder, panic disorder, Parkinson's disease, dementia, Alzheimer's disease, cognitive impairment, chemotherapy-induced cognitive dysfunction (“chemobrain”), Down syndrome, autism spectrum disorders, hearing loss, tinnitus, spinocerebellar ataxia, amyotrophic lateral sclerosis, multiple sclerosis, Huntington's disease, stroke, and disturbances due to radiation therapy, chronic stress, optic neuropathy or macular degeneration, or abuse of neuro-active drugs, selected from alcohol, opiates, methamphetamine, phencyclidine and cocaine.
Owner:F HOFFMANN LA ROCHE & CO AG
Benzopiperidine derivative, preparation method thereof and medical use thereof
ActiveUS20180318284A1ConstantLess side effectsOrganic active ingredientsNervous disorderDiseaseSelective receptor modulator
The present invention relates to a benzopiperidine derivative, a preparation method thereof and a medical use thereof. In particular, the present invention relates to a benzopiperidine derivative as shown by general formula (I), a preparation method thereof and a pharmaceutical composition containing the derivative, as well as a use thereof as an estrogen receptor modulator in the prevention and / or treatment of estrogen receptor-mediated or dependent diseases or conditions. Preferably, the disease is breast cancer. The substituents in the general formula (I) are the same as those defined in the description.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
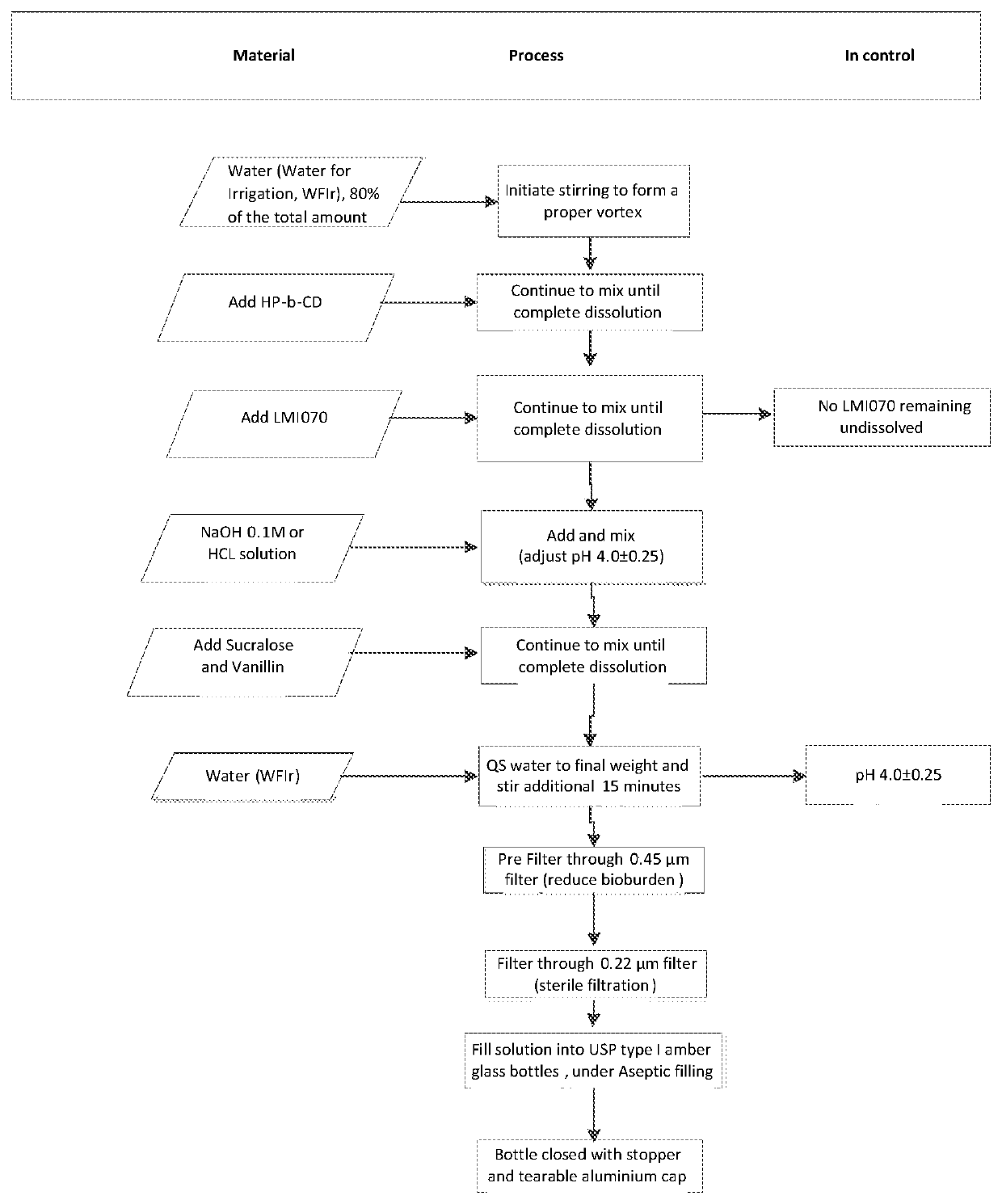
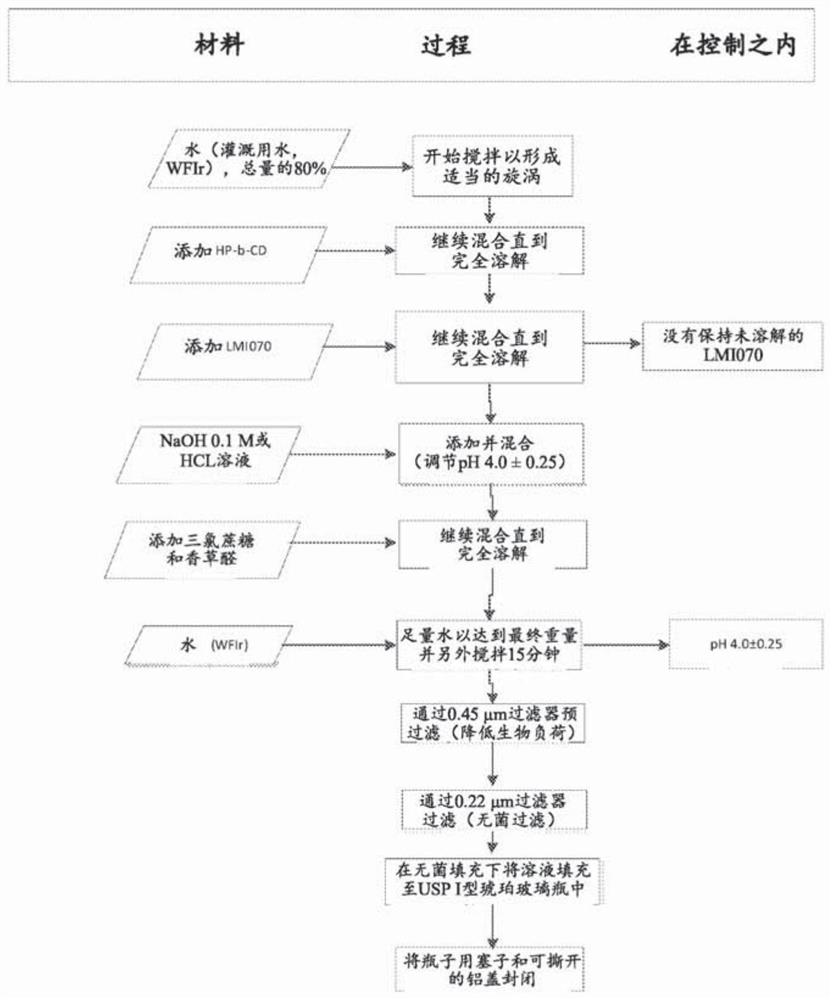
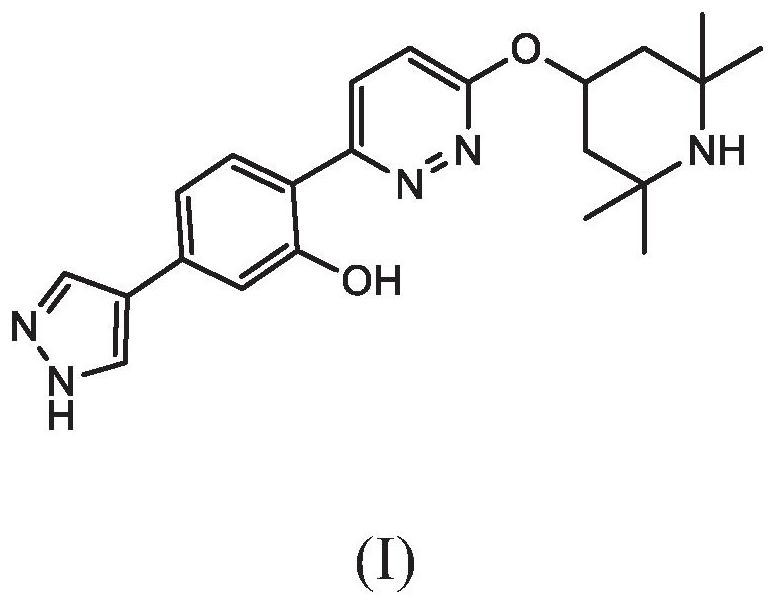
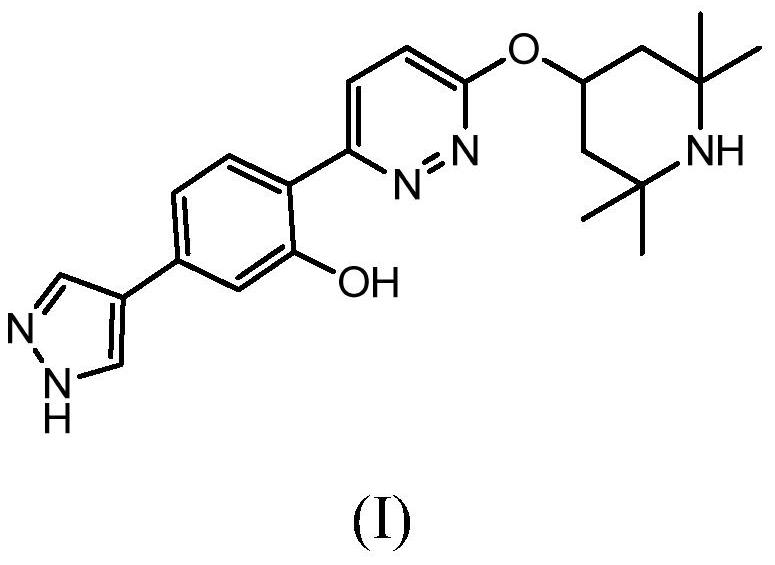
Oral formulations of branaplam
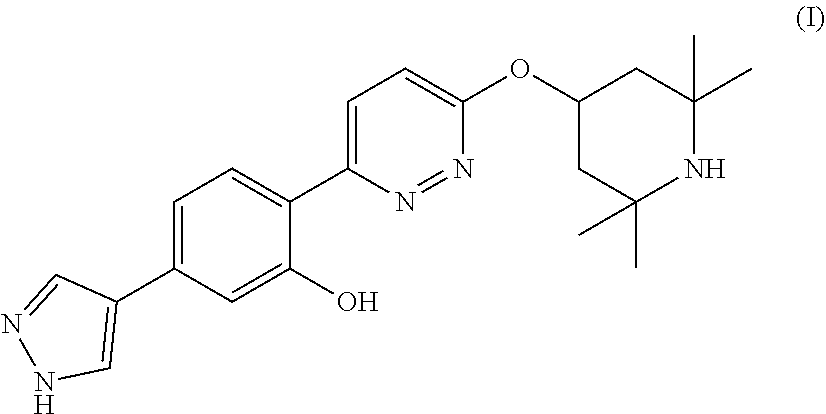
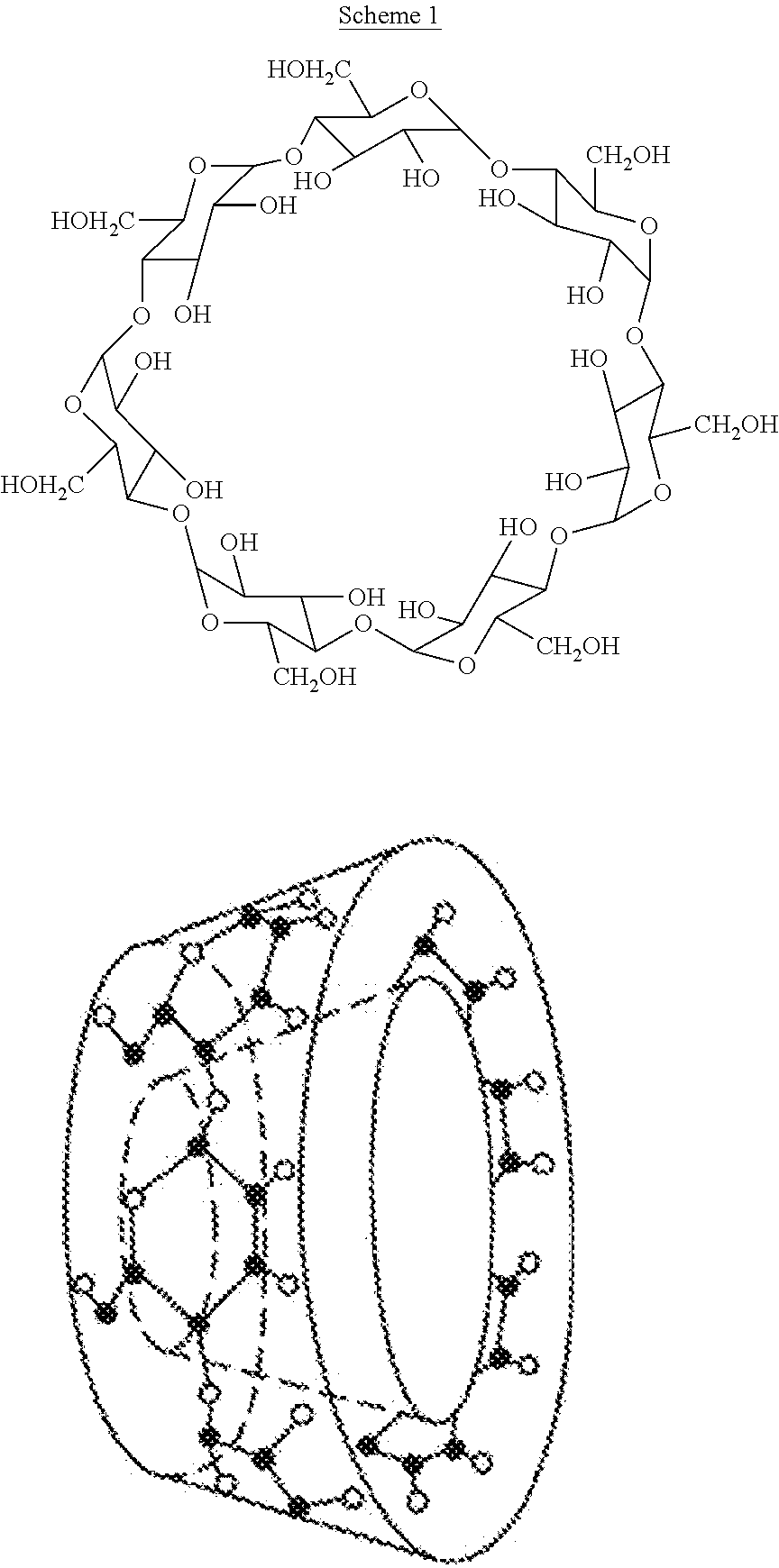
PendingUS20220071996A1Improve toleranceOrganic active ingredientsNervous disorderPyridazineOral medication
The present invention relates to pharmaceutical compositions suitable for oral administration comprising 5-(1H-pyrazol-4-yl)-2-(6-((2,2,6,6-tetramethylpiperidin-4-yl)oxy)pyridazin-3-yl)phenol (branaplam) and a pharmaceutically acceptable cyclodextrin.
Owner:NOVARTIS AG
Pipecolinic acid 4-hydroxylase and method for producing 4-hydroxy amino acid using same
ActiveUS20180282709A1Efficient productionHigh optical purityBacteriaOxidoreductasesPipemidic acidGlutaric acid
The present invention provides a pipecolic acid 4-hydroxylase protein exemplified by the following (A), (B), and (C), having activity to react with L-pipecolic acid in the presence of 2-oxoglutaric acid and iron(II) ions to produce trans-4-hydroxy-L-pipecolic acid, and a method for producing 4-hydroxy amino acid, which method comprises reacting the pipecolic acid 4-hydroxylase protein, cells containing the protein, a treated product of the cells, and / or a culture liquid obtained by culturing the cells, with α-amino acid to produce 4-hydroxy amino acid:(A) a polypeptide comprising the amino acid sequence represented by SEQ ID NO:2, 4, 6, 8, 10, 12, 16, or 18;(B) a polypeptide comprising the amino acid sequence represented by SEQ ID NO:2, 4, 6, 8, 10, 12, 16, or 18 except that one or several amino acids are deleted, substituted, and / or added, and having pipecolic acid 4-hydroxylase activity; and(C) a polypeptide having an amino acid sequence that is not less than 80% identical to the amino acid sequence represented by SEQ ID NO:2, 4, 6, 8, 10, 12, 16, or 18, and having pipecolic acid 4-hydroxylase activity.
Owner:API CORP (JP)
1-(pyrimidin-4-yl) 3-aminopiperidine derivatives and their preparation methods and uses
The invention relates to the field of chemical medicines and concretely relates to a 1-(pyrimidine-4-yl)-3-aminopiperdine derivative and its preparation method and use. The invention provides the 1-(pyrimidine-4-yl)-3-aminopiperdine derivative. The 1-(pyrimidine-4-yl)-3-aminopiperdine derivative has a structure shown in the formula IV. The invention also provides a preparation method and a use of the 1-(pyrimidine-4-yl)-3-aminopiperdine derivative. The 1-(pyrimidine-4-yl)-3-aminopiperdine derivative can selectively inhibit JAK3 and provide a novel choice for treating JAK3-related diseases such as rheumatoid arthritis, asthma, chronic obstructive pulmonary diseases and tumors.
Owner:CHENGDU ZENITAR BIOMEDICAL TECH CO LTD
Oral formulations of branaplam
The present invention relates to pharmaceutical compositions suitable for oral administration comprising 5-(1H-pyrazol-4-yl)-2-(6-((2,2,6,6-tetramethylpiperidin-4-yl)oxy)pyridazin-3-yl)phenol (branaplam) and a pharmaceutically acceptable cyclodextrin.
Owner:NOVARTIS AG
Pipecolinic acid preparation process based on reverse dropping
InactiveCN110218176AEnhanced convectionPrevent splashOrganic chemistryAcid preparationsMixing effect
The invention discloses a pipecolinic acid preparation process based on reverse dropping and belongs to the technical field of medicines. By adopting the pipecolinic acid preparation process based onreverse dropping, S1-S2 and S3-S7 can be implemented simultaneously, so that the lasting time of the whole preparation process can be effectively shortened; meanwhile, by adopting a dropping mode in the preparation process, a conventional mode of dropping from top to bottom is changed into a mode of dropping from bottom to top, small water domes can be formed because of instantaneous impact resulted when a liquid to be dropped is fed into a reaction kettle, and are diffused outwards and upwards, liquid convection of the dropped liquid and a reactant can be enhanced because of the water domes,thus a good mixing effect is achieved, reactions can be accelerated to a certain extent, the preparation efficiency can be improved, and compared with the mode of dropping from top to bottom, the process is capable of effectively avoiding the phenomenon that the content of the reactant is changed to a certain extent as the liquid is splashed because of water impact and is adhered to an inner wall.
Owner:BTC PHARMA TECH CO LTD
Arylethyl piperidinyl derivatives and their application in the treatment of schizophrenia
The invention discloses an arylethyl piperidinyl derivative and its application in anti-schizophrenia. The results of pharmacological experiments show that the arylethylpiperidine compounds involved in the present invention, to dopamine D 2 、D 3 , 5‑HT 1A , 5‑HT 2A The receptor has a higher affinity for D 3 / D 2 Receptor selectivity is good. The results of in vivo experiments show that the preferred compound has good anti-schizophrenia effect, low acute toxicity, high safety, good pharmacokinetic properties, and has development value as a new antipsychotic and neurological disease drug with high efficiency and low toxicity. The arylethylpiperidine compound is a compound having the general structural formula (I) or its salt or a hydrate of the salt:
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Spiro piperidone derivative
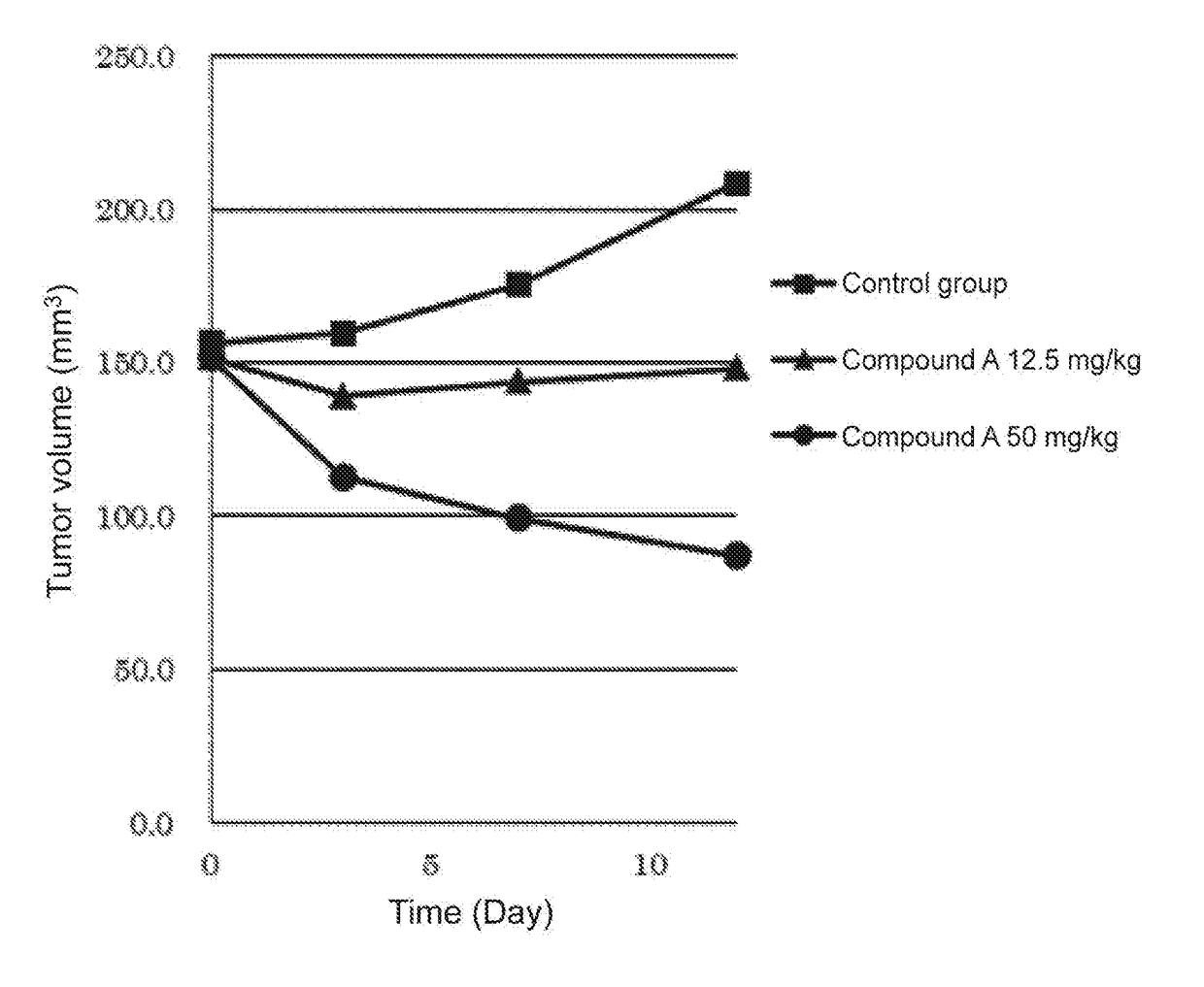
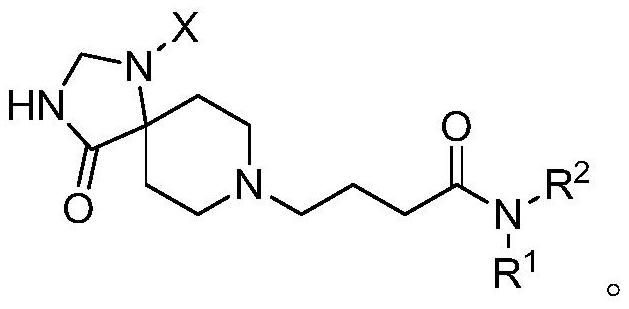
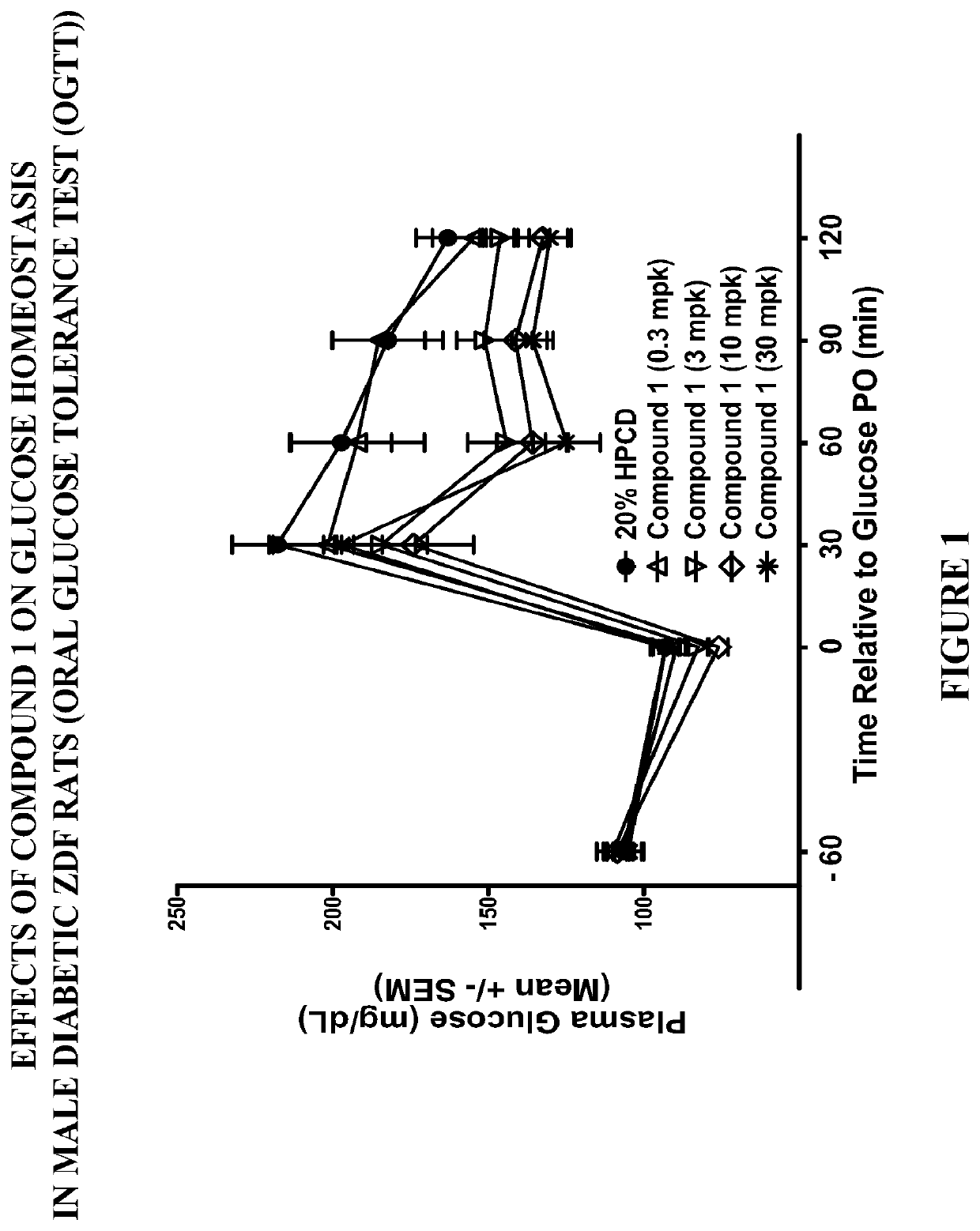
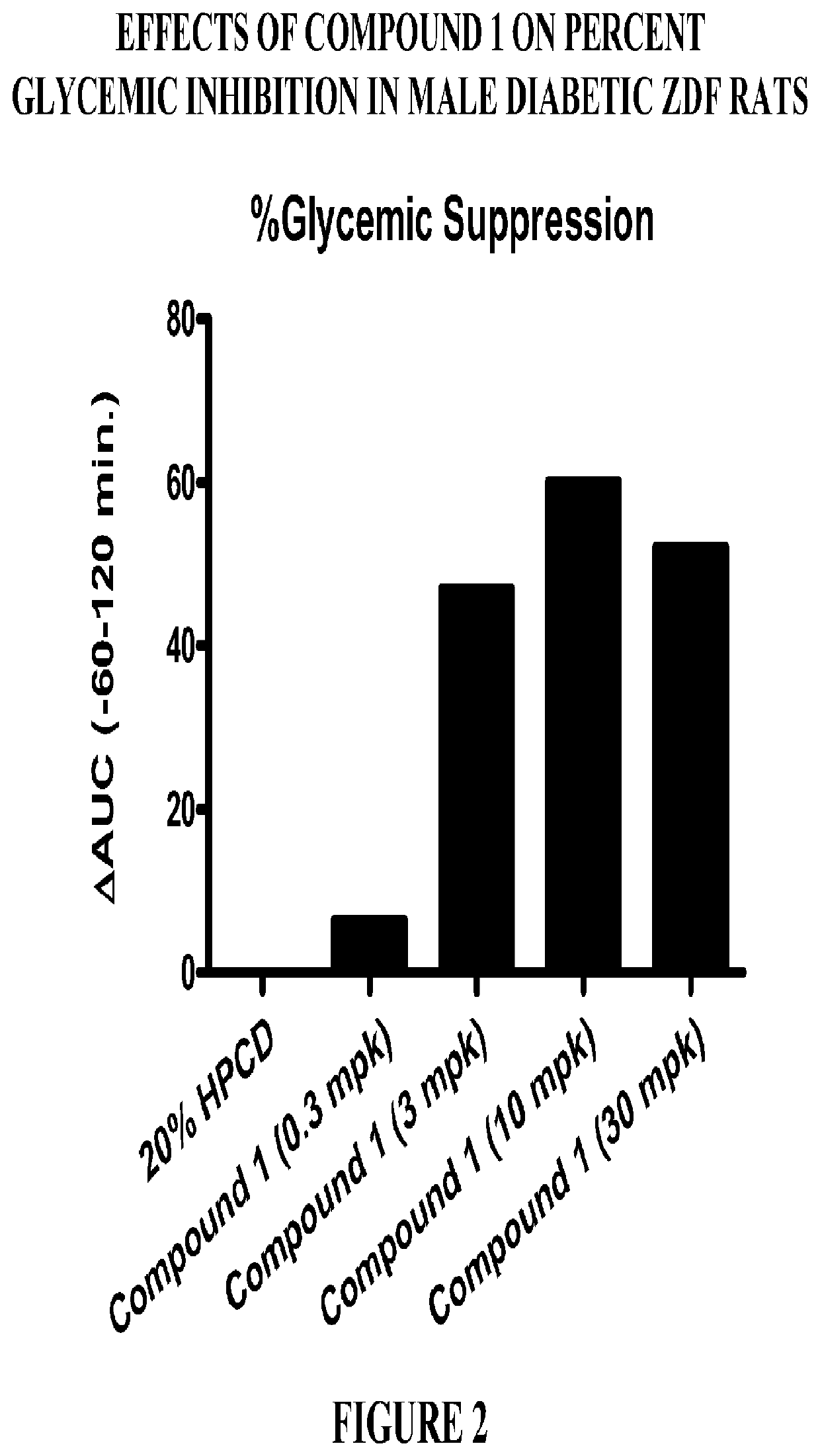
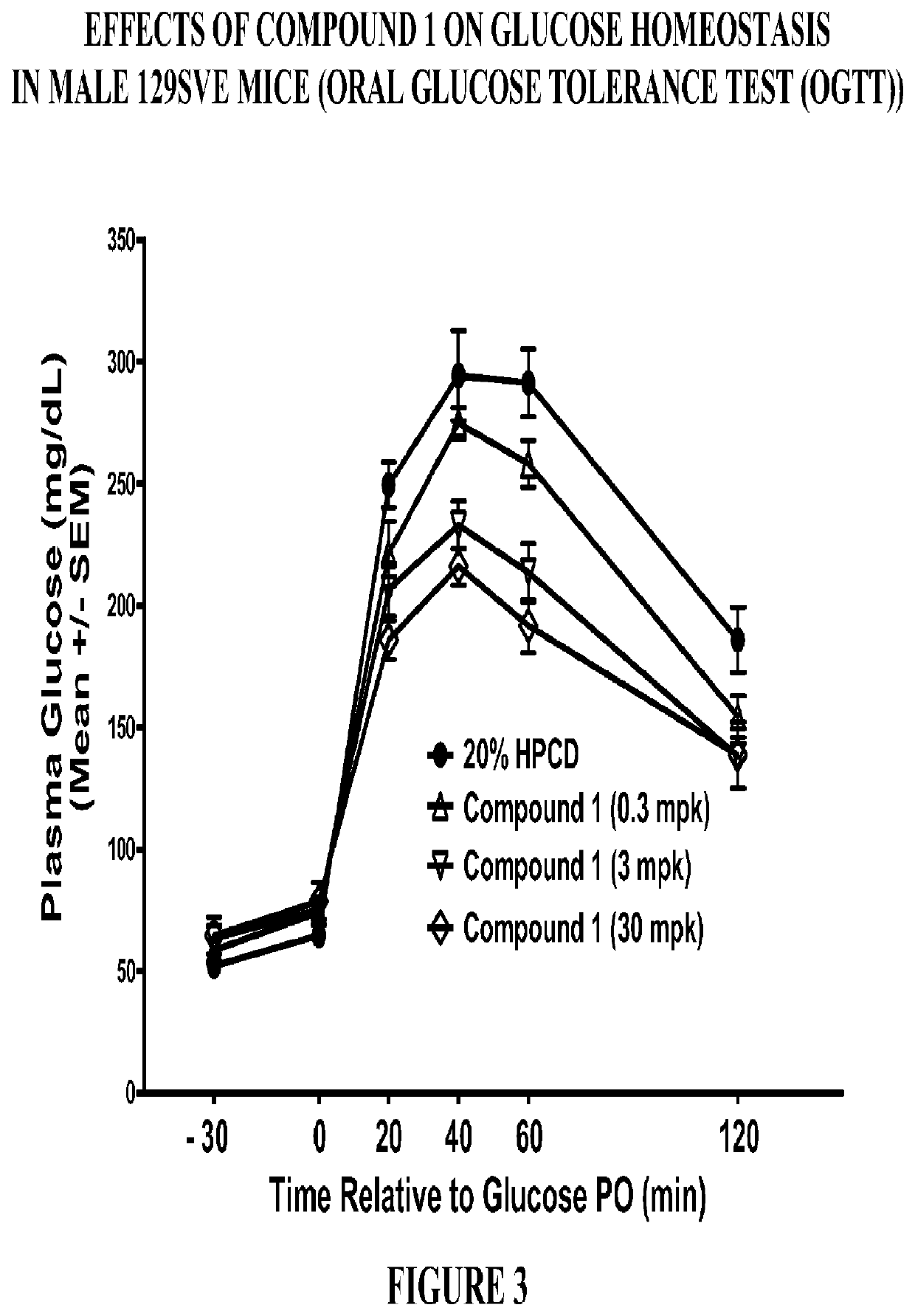
PendingCN113480536AGood antitumor activityPerformance selectivityOrganic chemistryAntineoplastic agentsPipequalinePharmaceutical drug
The invention relates to the technical field of medicines, in particular to a spiro piperidone derivative defined in the specification, wherein the pharmacological experiments show that the derivative or salt has very strong inhibitory activity on KRAS-PDE delta protein-protein interaction and has relatively strong in-vitro anti-tumor activity. The invention also provides a preparation method of the derivative and the pharmaceutically acceptable salt thereof, and an application of the derivative and the pharmaceutically acceptable salt thereof in preparation of a KRAS-PDE delta inhibitor and an antitumor drug.
Owner:THE NAVAL MEDICAL UNIV OF PLA
Modulators of the gpr119 receptor and the treatment of disorders related thereto
The present invention relates to the GPR119 receptor agonists: 3-fluoro-4-(5-fluoro-6-(4-(3-(2-fluoropropan-2-yl)-1,2,4-oxadiazol-5-yl)piperidin-1-yl)pyrimidin-4-ylamino)-N,N-dimethylbenzanide; -fluoro-4-(5-fluoro-6-(4-(3-(2-fluoro-propan-2-yl)-1,2,4-oxadiazol-5-yl)piperidin-1-yl)pyrimidin-4-ylamino)-N-methylbenzamide; and 3-fluoro-4-(5-fluoro-6-(4-(3-(2-fluoropropan-2-yl)-1,2,4-oxadiazol-5-yl)piperidin-1-yl)pyrimidin-4-ylamino)benzamide, and pharmaceutically acceptable salts, solvates, and hydrates thereof, that are useful as a single pharmaceutical agent or in combination with one or more additional pharmaceutical agents, such as, a DPP-IV inhibitor, a biguanide, an alpha-glucosidase inhibitor, an insulin analogue, a sulfonylurea, an SGLT2 inhibitor, a meglitinide, a thiazolidinedione, or an anti-diabetic peptide analogue, in the treatment of, for example, a disorder selected from: a GPR119-receptor-related disorder; a condition ameliorated by increasing secretion of an incretin; a condition ameliorated by increasing a blood incretin level; a condition characterized by low bone mass; a neurological disorder; a metabolic-related disorder; type 2 diabetes; obesity; and complications related thereto.
Owner:ARENA PHARMA
Pharmacophores, compounds and methods having application in the treatment of cancer through inhibition of cyp17a1 and cyp19a1
The invention provides compounds for use as medicaments, which act by inhibiting CYP17A1 and CYP19A1 enzymes. The compounds have particular application in the treatment of cancer especially prostate cancer and breast cancer. The compounds have the formula: [Chem. 1] wherein: R is independently selected from the group consisting of optionally substituted arylamide; optionally substituted alkylarylamide; optionally substituted aryl carboxamide; optionally substituted cyanopiperidine; optionally substituted oxopiperidine; optionally substituted N-(pyridin-3-yl); optionally substituted pyridin-3-yl; optionally substituted pyrazole-4-carboxamide; optionally substituted pyrimidin-4-ylcarboxamide; optionally substituted pyrimidin-4-ylcarboxamide; optionally substituted 1H-pyrrol-2-ylcarboxamide; optionally substituted morpholin carboxamide; optionally substituted 1H-indazol-3-ylcarboxamide; optionally substituted 5-cyanopiperidin-3-ylcarboxamide; optionally substituted quinolin-7-yl; optionally substituted pyrazin-2-ylcarboxamide; optionally substituted 1H-1,3-benzodiazole-6-carboxamide; and optionally substituted 3-oxo-3,4-dihydro-2H-1,4-benzoxazin-7-ylcarboxamide; Each R1, R2, R3, R4, R5 is independently selected from the group consisting of H; OH; a halogen atom; OCH3; and NH2; and X is independently selected from the group consisting of O, H and OH. Some of the compounds are claimed per se and the invention also encompasses pharmaceutically acceptable salts, solvates, hydrates, primary metabolites and prodrugs thereof.
Owner:MANGOSUTHU UNIV TECH
4-hydroxypiperidine derivatives and their use as inhibitors of ubiquitin specific protease 19 (USP19)
PendingUS20210070773A1Increased caspase activationLower Level RequirementsOrganic chemistryMetabolism disorderAtrophyPipequaline
Inhibitors of ubiquitin specific protease 19 (USP19) of Formula (I) are provided, together with pharmaceutical compositions comprising said inhibitors, and methods of use thereof. The compounds can be used in in the treatment of muscular atrophy, obesity, insulin resistance or type II diabetes or in reducing the loss of muscle mass.
Owner:ALMAC DISCOVERY LIMITED
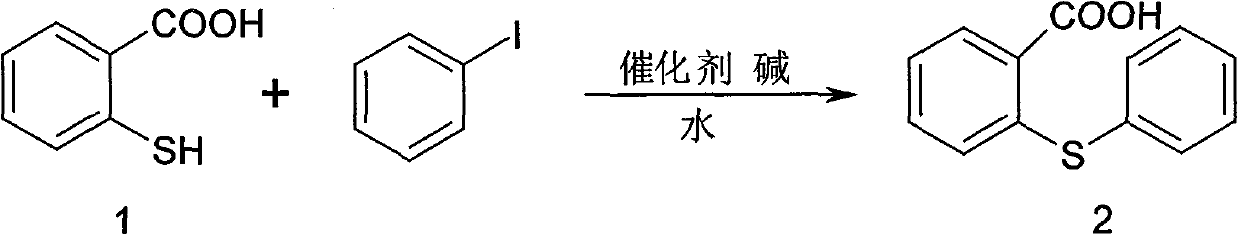
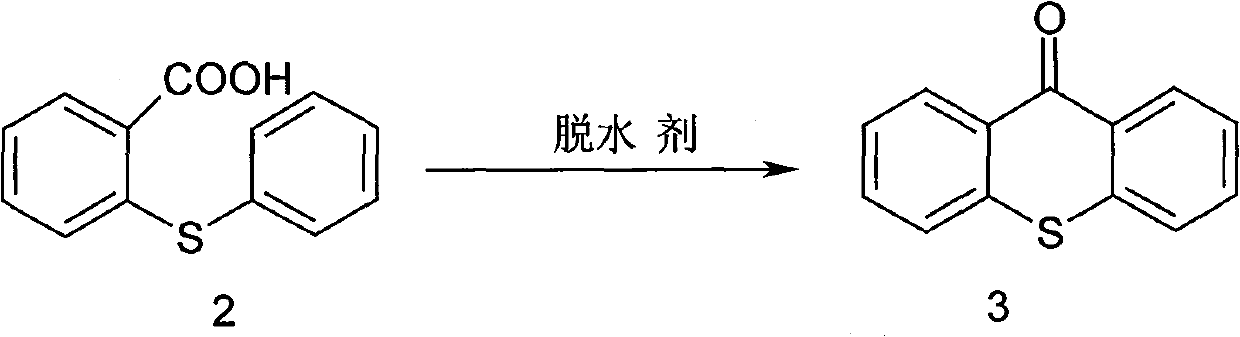
Synthesis method of metixene hydrochloride
InactiveCN101525333BWide variety of sourcesLow priceNervous disorderOrganic chemistryBenzoic acidAntiparkinsonian drug
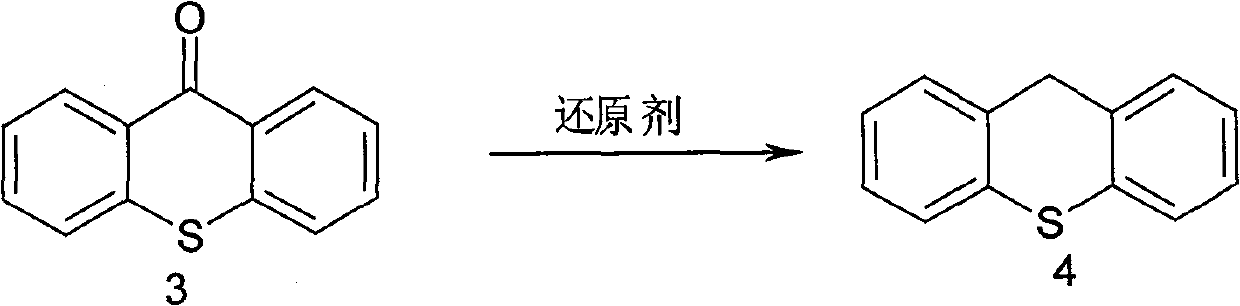
A synthesis method of metixene hydrochloride relates to a synthesis process of an antiparkinsonian drug. The synthesis method comprises the following steps: taking thiosalicylic acid as a raw material to obtain 2-thiophenyl benzoic acid by coupling with iodobenzene; then dehydrating and cyclizing to obtain 9-thioxanthone; reducing a carbonyl to obtain thioxanthene; and finally allowing the thioxanthene to react with 3-chloromethyl-1-pipecoline to obtain the target product (5) metixene hydrochloride shown as a formula (5) below. The synthesis method has the advantages of available raw materials, low cost, short preparation course, simple operation and easy industrialization, and helps produce the metixene hydrochloride which is the antiparkinsonian drug.
Owner:EAST CHINA NORMAL UNIV
4,4-diphenylpiperidine compounds or pharmaceutically acceptable salts thereof, pharmaceutical compositions and uses thereof
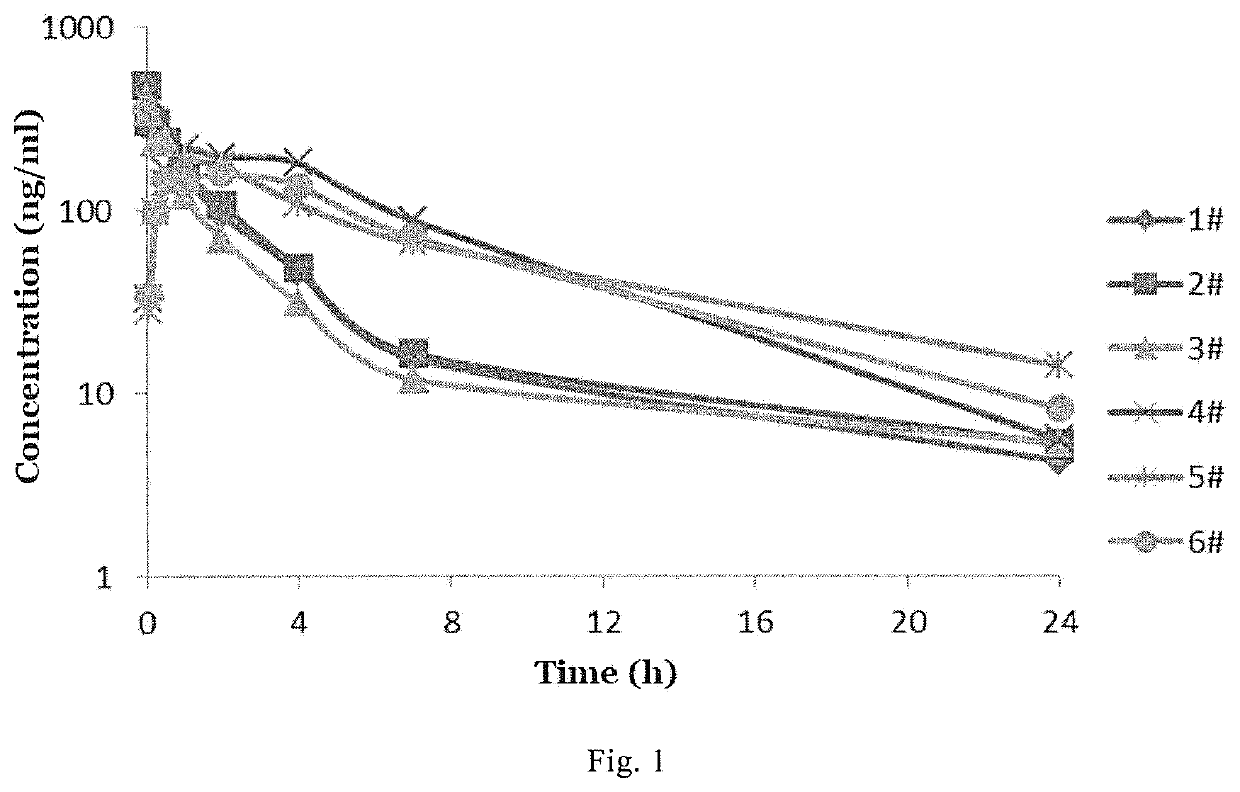
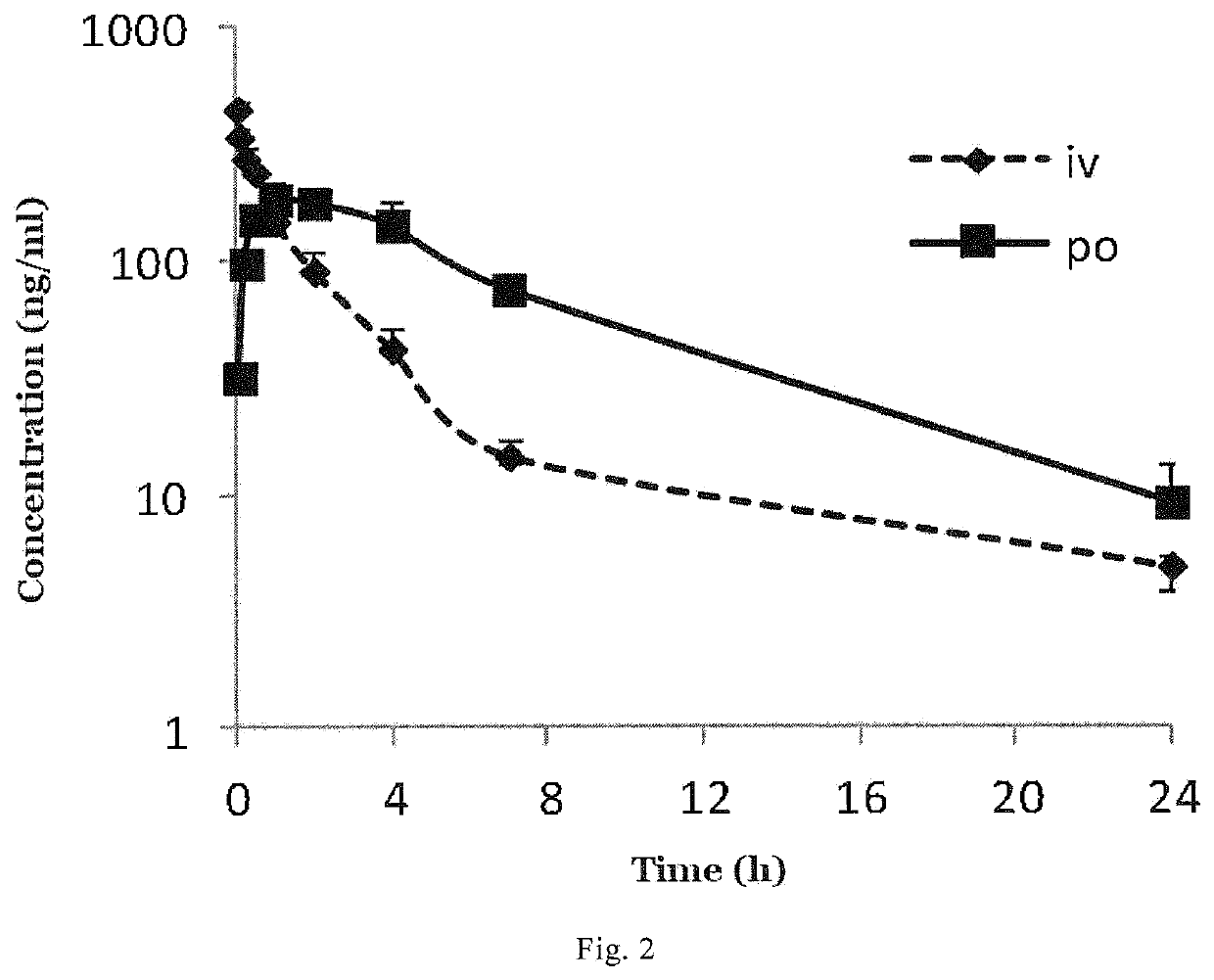
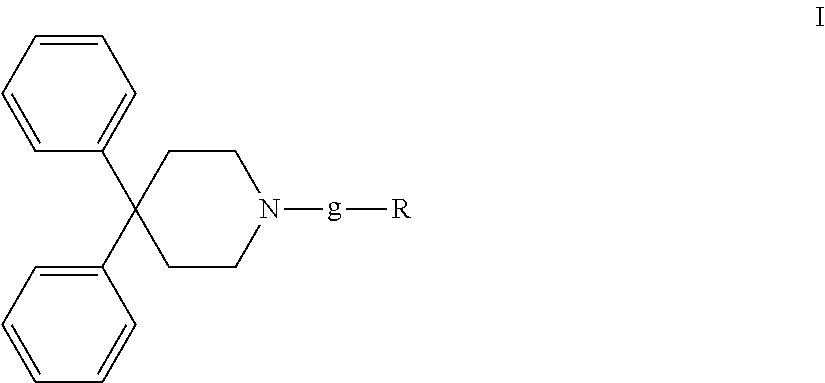
ActiveUS10889575B2Improved pharmacokinetic propertiesEffectively painOrganic active ingredientsNervous disorderPipequalinePharmaceutical medicine
The invention belongs to the field of medicine and chemical industry and relates to a 4,4-diphenylpiperidine compound or a pharmaceutically acceptable salt thereof, a pharmaceutical composition comprising the same and uses thereof. In particular, the invention relates to a compound of Formula I or a pharmaceutically acceptable salt thereof, and to a pharmaceutical composition comprising the compound or a pharmaceutically acceptable salt thereof. In the present invention, the compound or pharmaceutically acceptable salt thereof and the pharmaceutical composition have significant activity in blocking an N-type calcium channel, and have good pharmacokinetic properties, can effectively relieve pain, and have a potential as a new medicament for prevention or treatment of pain, stroke, cerebral ischemia, alcohol addiction, alcoholism, kidney disease, addictive disorder caused by analgesic or tolerance disorder caused by analgesic.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com