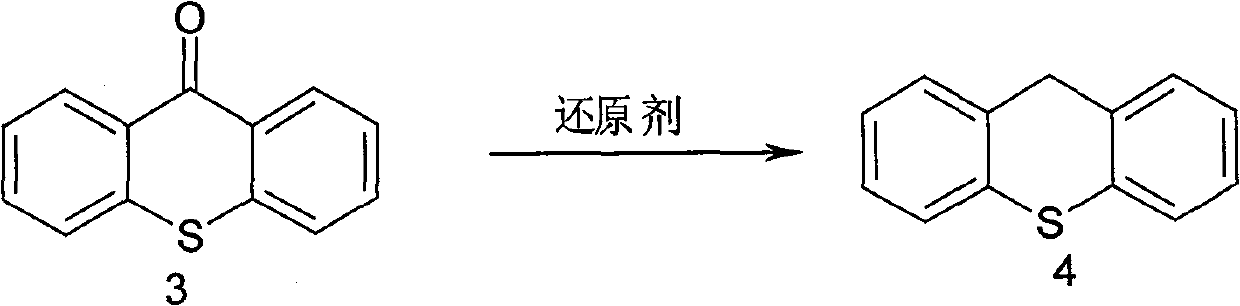
Synthesis method of metixene hydrochloride
A technology of Mesaisan hydrochloride and a synthesis method, which is applied in the field of synthesis technology of anti-shock paralysis drugs, can solve the problems of high price of molybdenum trisulfide, does not conform to green chemistry, is unfavorable to industrialization, and achieves low cost, low price and production cost. reduced effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
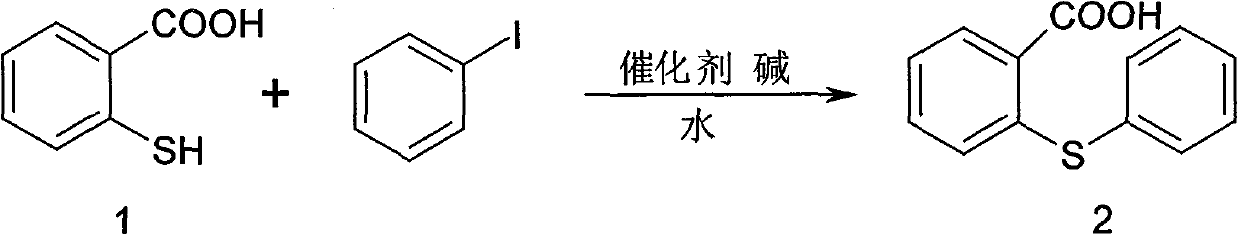
[0030] Synthesis of 2-phenylthiobenzoic acid (2)
[0031] KOH (54.5g, 972.7mmol) and 600ml H 2 O was made into a solution and cooled to room temperature. N 2 Under protection, the raw material thiosalicylic acid (30g, 389.1mmol) was added into a 1000ml three-neck flask, dissolved in the KOH solution prepared above and heated to 60°C and stirred for 30 minutes, N 2 Add cuprous bromide (1.675g, 11.6mmol) under protection, add iodobenzene, heat up to reflux, react for 20h, stop heating, cool the reaction solution to room temperature, add 100ml of concentrated hydrochloric acid dropwise, the time is 30min, and the pH value is 1 , a large number of yellow solids were generated, and the yellow solid was obtained by suction filtration, and the precipitate was washed with 2 × 500ml of water to obtain a white solid, which was dried to obtain the target product 2-phenylthiobenzoic acid (2) 85.5g, yield 95.5%
[0032] 1 HNMR (500MHz, CDCl 3 )8.2~8.4(d, 1H), 7.7~7.8(t, 2H), 7.5~7.6(t...
Embodiment 2
[0043] Synthesis of 2-phenylthiobenzoic acid (2)
[0044] KOH (54.5g, 972.7mmol) and 600ml H 2 O was made into a solution and cooled to room temperature. N 2 Under protection, the raw material thiosalicylic acid (1) (30g, 389.1mmol) was added into a 1000ml three-necked bottle, dissolved in the KOH solution prepared above and heated to 60°C and stirred for 30 minutes, N 2 Add cuprous iodide (2.2g, 11.6mmol) under protection, add iodobenzene, heat up to reflux, react for 20h, stop heating, cool the reaction solution to room temperature, add 100ml of concentrated hydrochloric acid dropwise, the time is 30min, and the pH value is 1 , a large number of yellow solids were generated, and the yellow solid was obtained by suction filtration, and the precipitate was washed with 2 × 500ml of water to obtain a white solid, which was dried to obtain the target product 2-phenylthiobenzoic acid (2) 85.5g, yield 95.5%
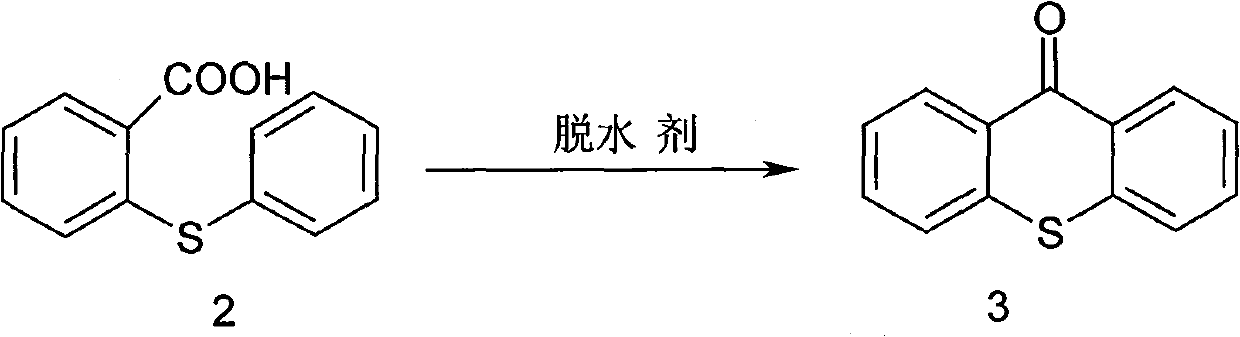
[0045] Synthesis of 9-thioxanthone (3)
[0046] N 2 Under protection...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com