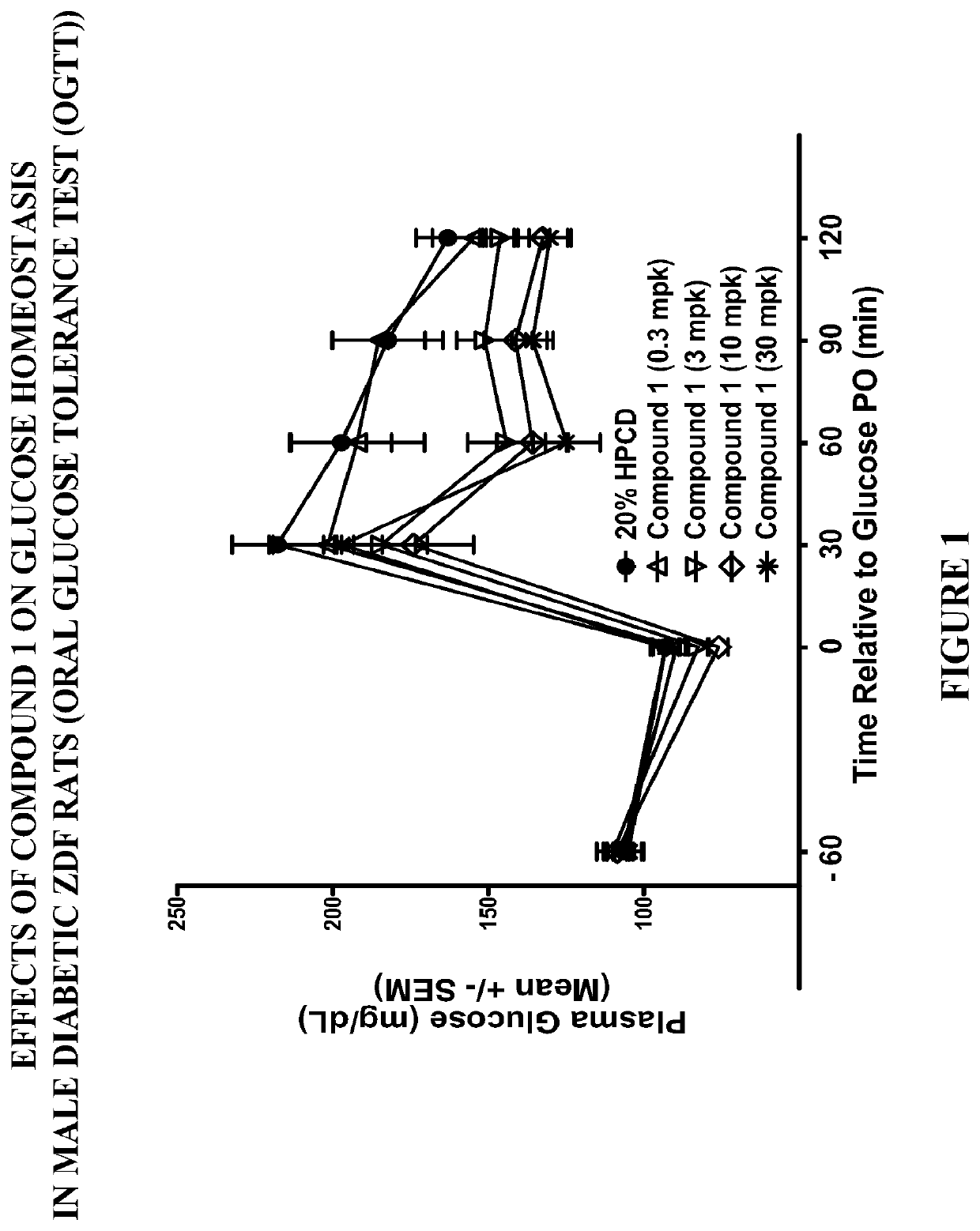
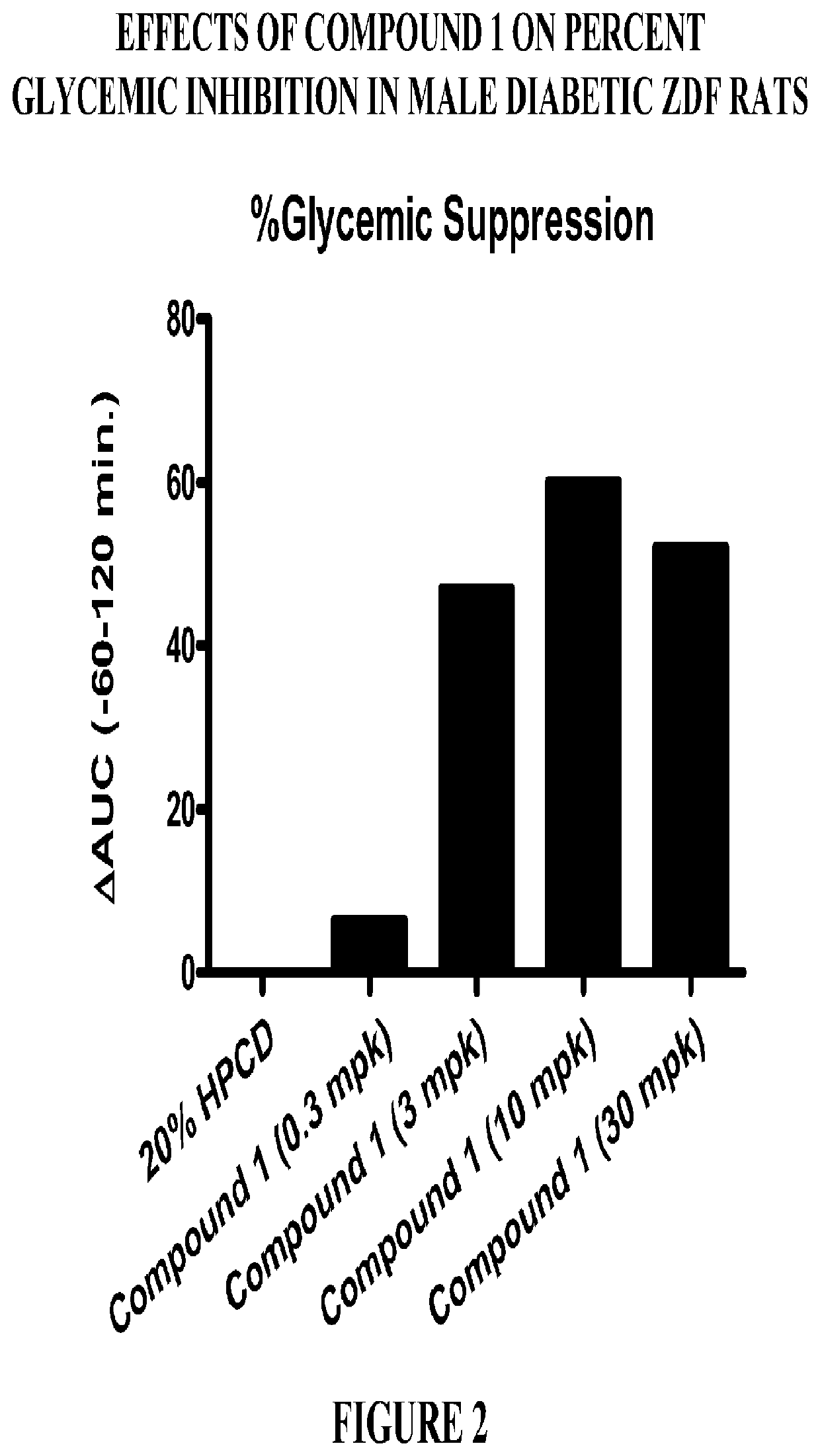
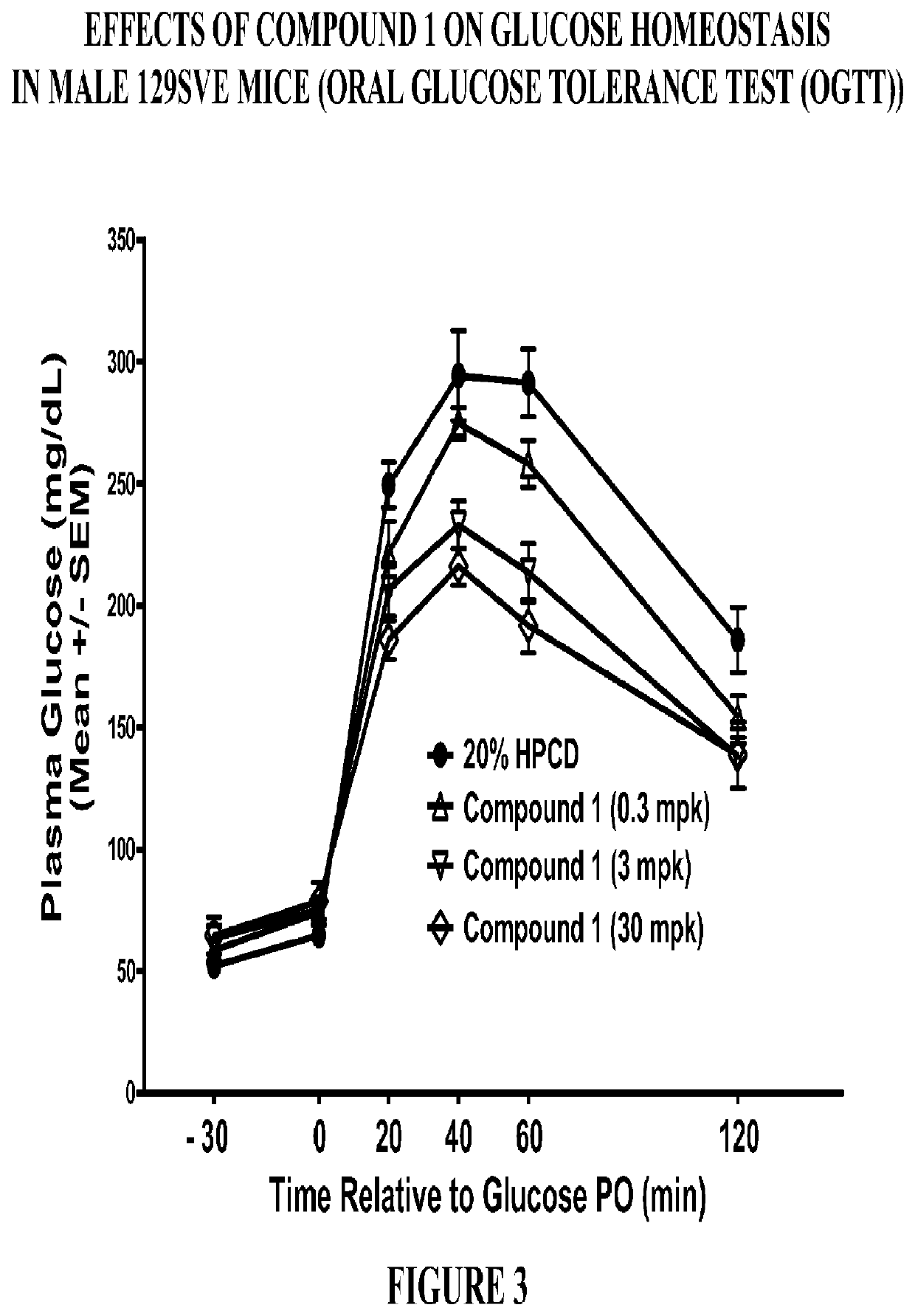
Modulators of the gpr119 receptor and the treatment of disorders related thereto
a technology of gpr119 receptor and gpr119 agonist, which is applied in the direction of drug composition, extracellular fluid disorder, metabolic disorder, etc., can solve the problems of not producing insulin, not efficiently using, and not moving glucose into their cells, so as to and increase the secretion of incretin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1.1
of 5-Fluoropyrimidine-4,6-diol
Method A
[0431]To a three-neck round-bottom flask equipped with an overhead stirrer, nitrogen flow, and reflux condenser, was added 25 wt % sodium methoxide in methanol (950 mL, 4.15 mol) and formamide (357 mL, 8.98 mol). The mixture was heated to about 64° C. To the reaction mixture was added diethyl 2-fluoromalonate (177 mL, 1.12 mol) using an addition funnel over 1 h. The reaction temperature was maintained at 64° C. for 72 h. The reaction mixture was allowed to cool to room temperature and the solvent was removed under reduced pressure. The residue was cooled to 0° C. and slowly acidified with concentrated hydrochloric acid to pH 1-2 resulting in the precipitation of the product. The product was filtered and washed with an ice cold aqueous 1 N HCl solution. The off-white solid was suspended in acetonitrile, filtered and dried in a vacuum oven to give 5-fluoropyrimidine-4,6-diol (170 g, 1.31 mol) as a light brown-pinkish solid which was used without f...
example 1.2
of 4,6-Dichloro-5-fluoropyrimidine
Method A
[0433]To a 500 mL three-neck round-bottom flask containing phosphorus oxychloride (45.3 mL, 487 mmol) was slowly added 5-fluoropyrimidine-4,6-diol (20.0 g, 154 mmol) and the resulting reaction mixture was heated to 60° C. To the resulting slurry was slowly added N,N-dimethylaniline (42.2 mL, 331 mmol) over 4 h using a syringe pump and the reaction mixture was stirred at 60° C. for 16 h. The reaction mixture was cooled to room temperature and slowly added into a mixture of brine and ice (400 ml) with stirring. The aqueous layer was extracted with dichloromethane (2×250 mL). The combined organic layers (light amber) were washed with cold aqueous 6 N HCl solution (200 mL), dried over sodium sulfate, and filtered through a glass fiber paper by vacuum filtration and the solvent was removed under reduced pressure (no heat) to give 4,6-dichloro-5-fluoropyrimidine (13 g, 78 mmol, 50.6% yield) as an amber oil. 1H NMR (400 MHz, CDCl3) δ ppm 8.62 (s, 1...
example 1.3
of 2-Fluoro-2-methylpropanenitrile
Method A
[0435]To a 1 L three-neck round-bottom flask containing 2-hydroxy-2-methylpropanenitrile (120 mL, 1.31 mol) at 4° C. was slowly added (diethylamino)sulfur trifluoride (DAST) (172 mL, 1.31 mol) over 1 h via an additional funnel. The reaction mixture was allowed to warm to room temperature and stirred overnight. The mixture was directly purified by vacuum distillation (40-45° C. / 45 mmHg) to provide 2-fluoro-2-methylpropanenitrile (83.36 g, 0.957 mol, 73.0% yield) as a colorless oil containing methacrylonitrile (˜10%). 1H NMR (400 MHz, CDCl3) δ ppm 1.77 (d, J=20 Hz, 6H).
Method B
[0436]2-Hydroxy-2-methylpropanenitrile (CAS #75-86-5, 221 mL, 2.420 mol) was cooled down to −10° C. (ice / acetone / dry ice) in a 1 L three necked round bottomed flask and DAST (246 mL, 1.861 mol) was added slowly using an addition funnel over a period of 2 h. Once the addition was finished, the reaction was allowed to warm up to room temperature and stirred overnight. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com