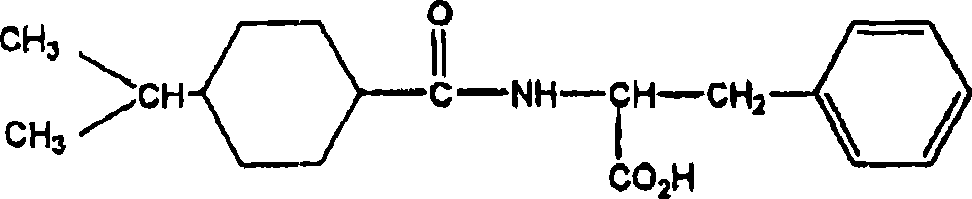
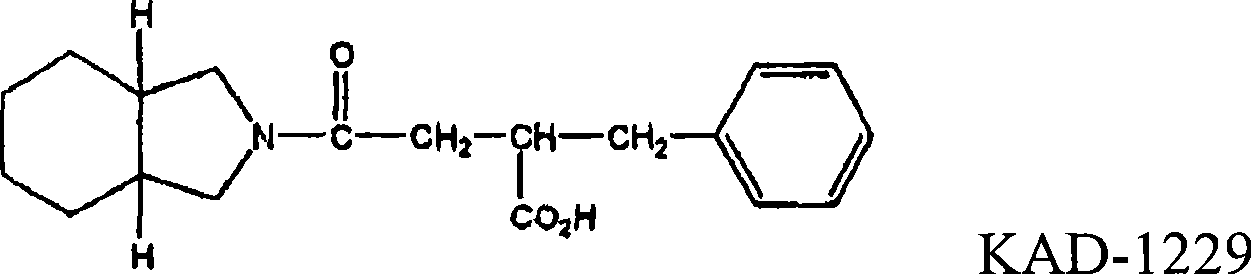
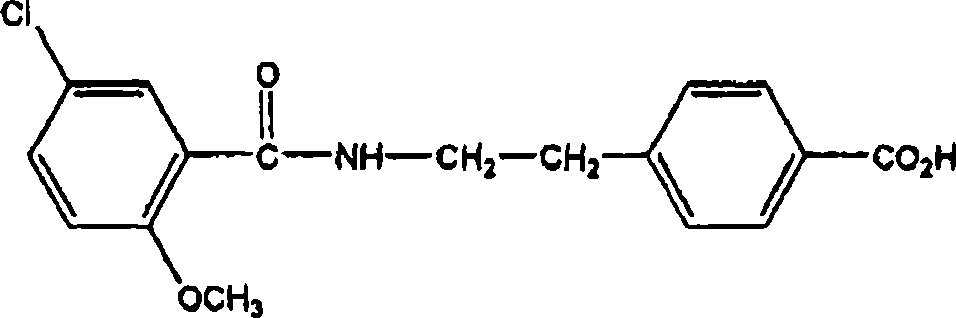Pharmaceutical composition comprising meglitinide for prevention of hepatic fibrosis
A pharmaceutical composition, technology of liver fibrosis, applied in the field of pharmaceutical composition for prevention, improvement or treatment of liver fibrosis, hepatocyte degeneration or liver cirrhosis, which can solve the problems of non-existence of quick effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0047] Fatty liver is characterized by abdominal ultrasound diagnosis or abdominal CT examination; various viral markers and autoantibody tests are negative; alcohol drinking experience and drug experience can be excluded; liver biopsy according to Brunt's criteria (Am J Gastroenterol.1999 94:2467- 2474) were diagnosed as NASH; in addition, 5 patients diagnosed with diabetes who could not obtain therapeutic effects from food and exercise therapy were the subjects. At the same time, the test method passed the ethical review of the clinical facility, and the informed consent of all subjects was obtained. For these 5 patients, 90 mg of nateglinide was administered orally three times a day before meals for 16 weeks. In the 0th week and the 12th week or 16th week, as an index of diabetes, the fasting blood sugar level, the blood sugar level of the glucose tolerance test 2 hours, the hemoglobin Alc value, and the liver / spleen ratio based on CT as a liver evaluation of fatty liver H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com