2-((2-(3-aminopiperidine-1)-4-oxythiophene (3, 2-d) pyrimidine-3(4H)-methyl) polymorphic benzonitrile, and preparation method and pharmacological applications thereof
A technology of aminopiperidine and oxythiophene, which is used in drug combinations, active ingredients of heterocyclic compounds, metabolic diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
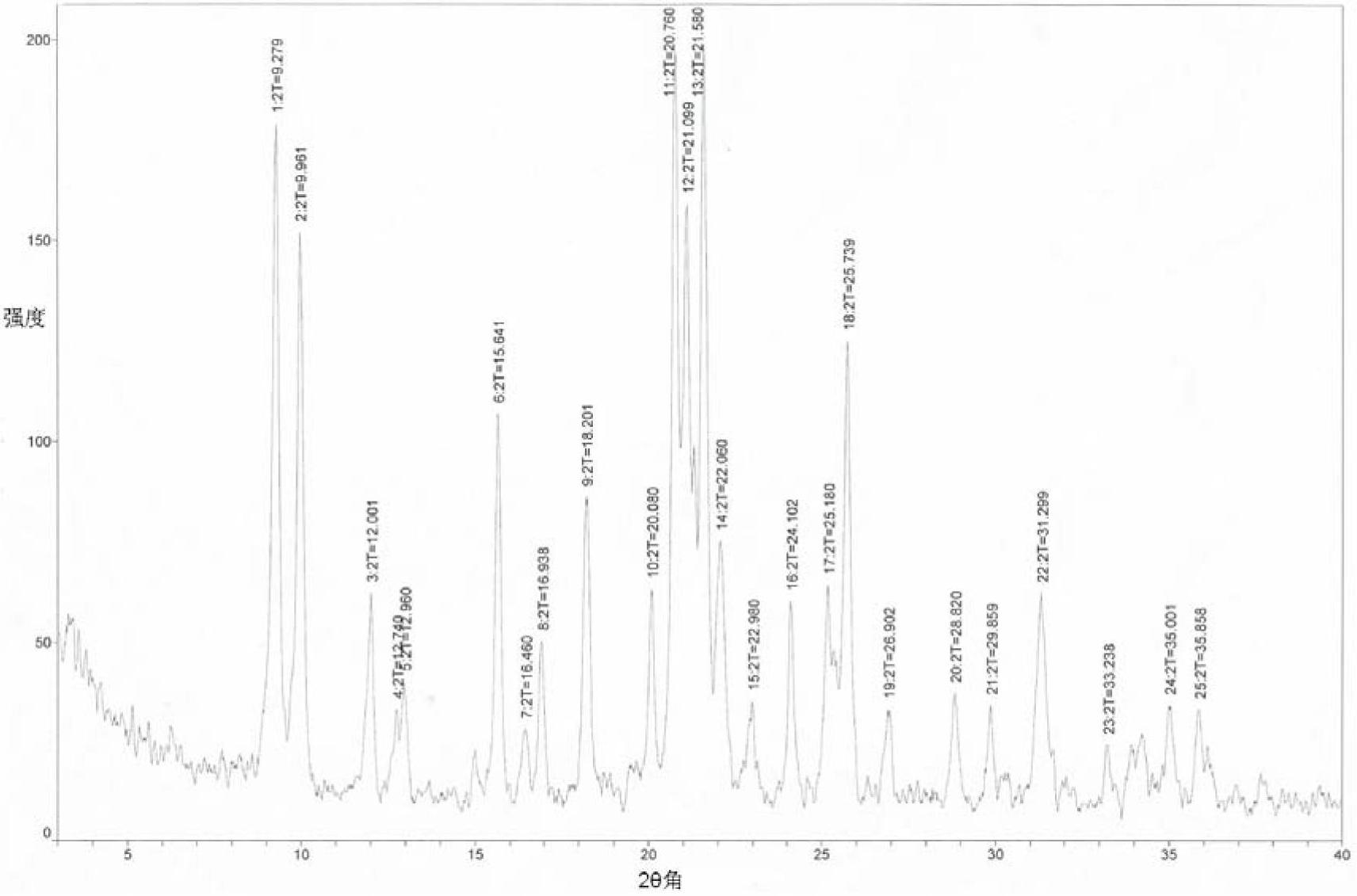
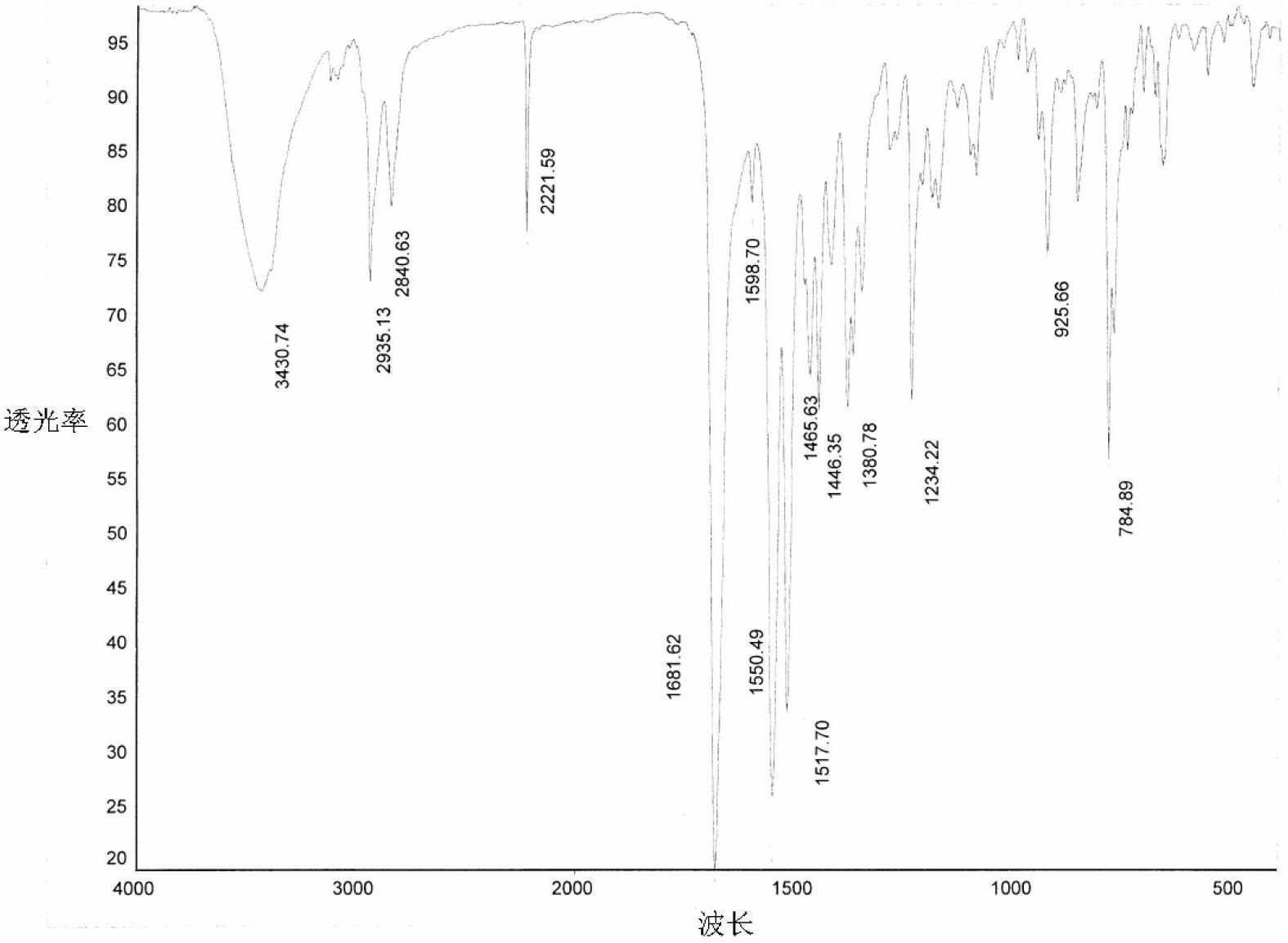
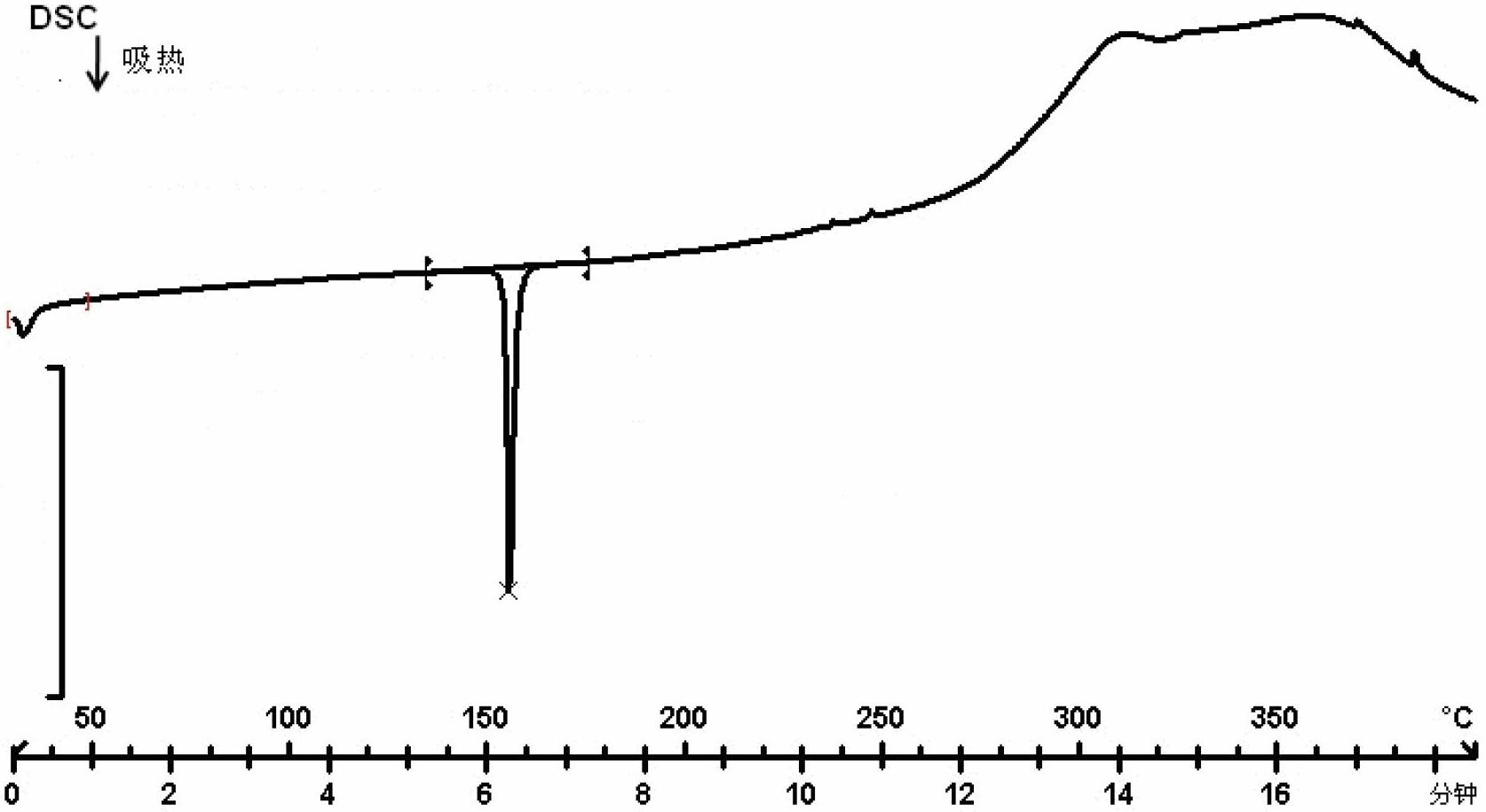
[0062] Example 1: Preparation of Form A of 2-((2-(3-aminopiperidine-1)-4-oxythieno[3,2-d]pyrimidine-3(4H)-methyl)benzonitrile
[0063] Dissolve 200 mg of 2-((2-(3-aminopiperidine-1)-4-oxothieno[3,2-d]pyrimidine-3(4H)-methyl)benzonitrile in 1 mL of ethyl acetate , add 3mL of petroleum ether, crystallize under stirring at room temperature, until no solid precipitates out, filter, place the obtained solid material in a vacuum drying oven, and vacuum dry for 70 hours at 25°C and 5KPa to obtain 2-(( 100 mg of 2-(3-aminopiperidine-1)-4-oxythieno[3,2-d]pyrimidine-3(4H)-methyl)benzonitrile A crystal.
Embodiment 2
[0064] Example 2: Preparation of Form A of 2-((2-(3-aminopiperidine-1)-4-oxythieno[3,2-d]pyrimidine-3(4H)-methyl)benzonitrile
[0065] Dissolve 200 mg of 2-((2-(3-aminopiperidine-1)-4-oxothiophene[3,2-d]pyrimidine-3(4H)-methyl)benzonitrile in 1 mL of ethanol, add 12mL of petroleum ether was crystallized under stirring at room temperature until no solid precipitated out, filtered, and the obtained solid material was placed in a vacuum drying oven, and vacuum-dried at 25°C and 5KPa for 70 hours to obtain 2-((2- (3-aminopiperidine-1)-4-oxythieno[3,2-d]pyrimidine-3(4H)-methyl)benzonitrile A crystal 110 mg.
Embodiment 3
[0066] Example 3: Preparation of Form A of 2-((2-(3-aminopiperidine-1)-4-oxythieno[3,2-d]pyrimidine-3(4H)-methyl)benzonitrile
[0067]Dissolve 200 mg of 2-((2-(3-aminopiperidine-1)-4-oxothieno[3,2-d]pyrimidine-3(4H)-methyl)benzonitrile in 1 mL of ethyl acetate , add 12mL of n-heptane, crystallize under stirring at room temperature, until no solid precipitates out, filter, place the resulting solid material in a vacuum drying oven, and vacuum dry for 70 hours at 25°C and 5KPa to obtain 2-( (2-(3-aminopiperidine-1)-4-oxythieno[3,2-d]pyrimidine-3(4H)-methyl)benzonitrile A crystal 100 mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com