Antitumor 3,5-diphenylmethylene-4-piperidone derivative and preparation method thereof
A technology of dibenzylidene and trifluoromethylbenzylidene, which is applied in the field of antineoplastic drugs and their preparation, achieves great application prospects, avoids genotoxicity, and has the effect of novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Synthesis of 3,5-bis(4-trifluoromethylbenzylidene)-4-piperidinone a
[0034] Mix 0.005 mol of 4-piperidone hydrochloride and 0.01 mol of 4-trifluoromethylbenzaldehyde in 12.5 mL of glacial acetic acid, pass through dry HCl gas at room temperature for 60 min, stir for 8 h, pass through a thin The end point of the reaction was determined by thin-layer chromatography (TLC) analysis. After completion of the reaction, suction filtration, the resulting precipitate was added to a mixture of 12.5 mL of potassium carbonate solution with a mass fraction of 25% and 12.5 mL of acetone solution, stirred at room temperature for 0.5 h, suction filtration, and recrystallized with a mixed solvent of methanol and chloroform to obtain 3, 5-bis(4-trifluoromethylbenzylidene)-4-piperidone 1.21g, yield 59%.
[0035]
Embodiment 2
[0037] Synthesis of N-(4-nitrobenzoyl)-3,5-bis(4-trifluoromethylbenzylidene)-4-piperidone b
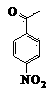
[0038]Mix 2 mL of 25% NaOH solution with 1 mmol of 3,5-bis(4-trifluoromethylbenzylidene)-4-piperidone in 5 mL of tetrahydrofuran solution, add 0.02 g of Tetrabutylammonium bromide with a fraction of 5% was stirred at 0-5°C for 15 min, 1.5 mmol 4-nitrobenzoyl chloride solution was added dropwise, and stirring was continued for 2 h. The end point of the reaction was determined by layer analysis, suction filtration, the residue was added to 10 mL of potassium carbonate solution with a mass fraction of 10%, stirred at room temperature for 2 h, the precipitate was collected, and recrystallized with methanol-chloroform mixed solvent to obtain N-(4-nitrobenzene Formyl)-3,5-bis(4-trifluoromethylbenzylidene)-4-piperidone 0.44g, yield 78%.
[0039]
Embodiment 3
[0041] Synthesis of N-(4-methylbenzenesulfonyl)-3,5-bis(4-trifluoromethylbenzylidene)-4-piperidone c
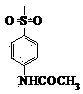
[0042] Mix 2 mL of 25% NaOH solution with 1 mmol of 3,5-bis(4-trifluoromethylbenzylidene)-4-piperidone in 5 mL of 1,2-dichloroethane Mix in medium, add 0.01 g of tetrabutylammonium chloride with a mass fraction of 5%, stir for 15 min at 0-5°C, add 1.5 mmol of 4-methylbenzenesulfonyl chloride solution dropwise, continue stirring for 5 h, pass through a thin Thin-layer chromatography (TLC) was used to determine the end point of the reaction, and 2 mol L -1 hydrochloric acid to adjust the pH to 1-4, collect the precipitate, and recrystallize with chloroform solvent to obtain N-(4-methylbenzenesulfonyl)-3,5-bis(4-trifluoromethylbenzylidene)-4 - piperidone 0.45g, yield is 79%.
[0043]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com