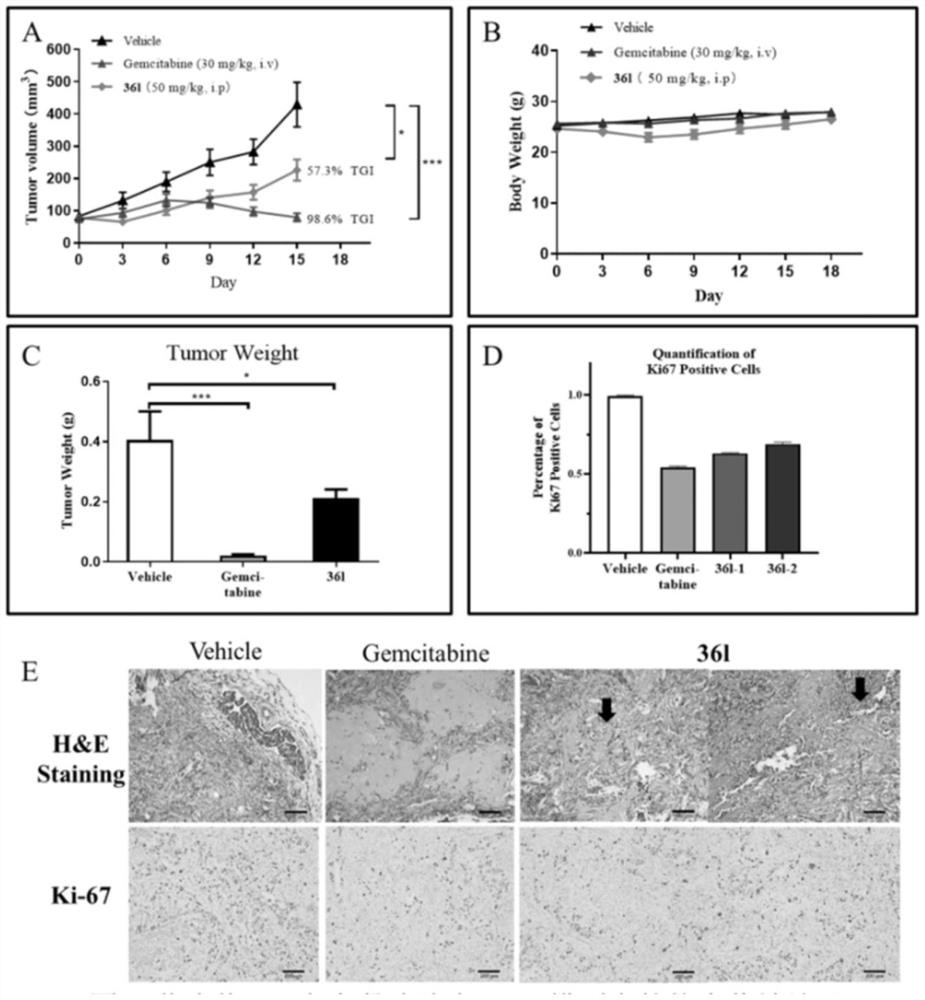
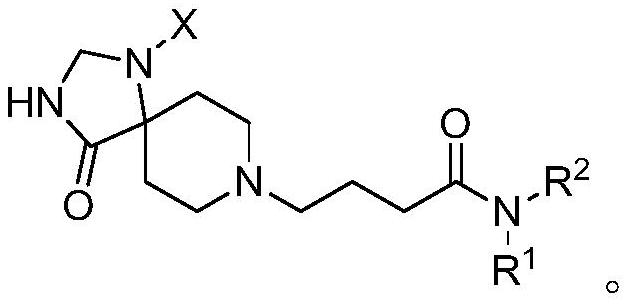
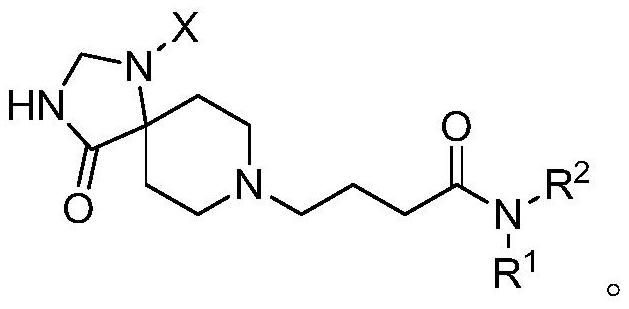
Spiro piperidone derivative
A technology of spirocyclic piperidone and derivatives, which is applied in the field of medicine and achieves the effect of good antitumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1: 1-Benzyl-4-(anilino)piperidine-4-carbonitrile (14)
[0058] Compounds 11 (3.0 g, 15.8 mmol) and 12 (1.48 g, 15.8 mmol) were weighed and dissolved in 20 mL of glacial acetic acid, then transferred to an ice bath, and compound 13 (4.7 g, 47.4 mmol) was slowly added dropwise after cooling. After the addition, transfer to room temperature for reaction. After 4 hours, the reaction was completed, and 2N NaOH solution was added to quench the reaction, and the pH was adjusted to 10. Then DCM was extracted (30 mL×3), the organic phases were combined, washed with saturated brine, spin-dried and slurried with ether to obtain the product. White solid, 2.8g, yield 60.9%.
Embodiment 2
[0059] Example 2: 1-Benzyl-4-(anilino)piperidine-4-carboxamide (15)
[0060] Compound 14 (2.8 g, 9.6 mmol) was weighed and dissolved in 14 mL of concentrated sulfuric acid, and reacted at room temperature. After 18 hours, the reaction was completed, and the reaction solution was added dropwise to 80 mL of 25% ammonia solution in an ice bath, and the pH was adjusted to above 10 after the addition was completed. Filter, retain the solid and dry it, and then beat with methanol to obtain the product. White solid, 2.2g, yield 94.2%.
Embodiment 3
[0061] Example 3: 1-Benzyl-4-(anilino)piperidine-4-carboxamide (16)
[0062] Weighed compound 15 (2.16g, 7mmol) and dissolved it in 15mL methanol, sonicated and transferred to 55°C for stirring, slowly added DMA (2.5g, 21mmol) dropwise, and then continued to react at 55°C. After 16 hours, the reaction was stopped and the reaction solution was spin-dried, and the crude product was slurried with ether to obtain the product. White solid, 1.53g, yield 68.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com