Metronidazole-isatin type compound as well as preparation method and application thereof
A compound, isatin-type technology, applied in the field of metronidazole-isatin-type compound and its preparation method and application, can solve problems such as difficult treatment of infectious diseases, and achieve good inhibitory and killing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
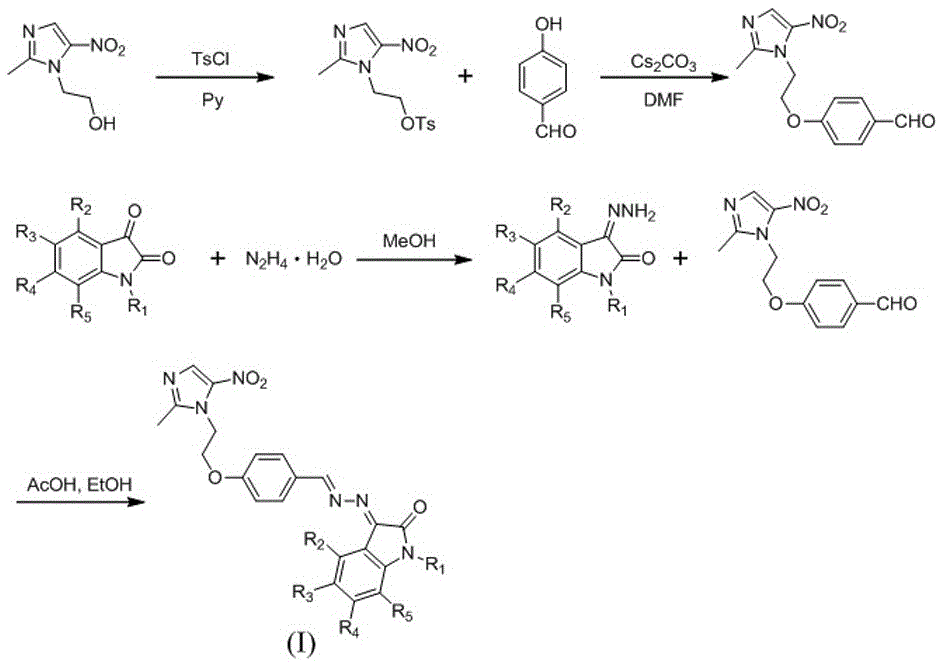
[0022] Example 1: Preparation of 2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethyl 4-methylbenzenesulfonate
[0023]
[0024] Place metronidazole (10.00mmol), pyridine (35.0mL) and p-toluenesulfonyl chloride (20.00mmol) in a round bottom flask, stir and react at room temperature for 12h, TLC shows that the reaction is complete, stop the reaction, and pour the reaction solution into an appropriate amount of In water, off-white crystals were precipitated, filtered with suction, washed the filter cake with an appropriate amount of water, and dried to obtain a white solid powder 2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethyl 4-methyl Base benzene sulfonate, productive rate 69.5%. 1 HNMR(d 6 -DMSO, 400MHz): δ:2.40(s,3H),2.41(s,3H),4.39(t,2H),4.55(t,2H),7.39(d,2H),7.59(d,2H), 7.92(s,1H);EIMSm / z=326[M + ].
Embodiment 2
[0025] Example 2: Preparation of 4-(2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethoxy)benzaldehyde
[0026]
[0027] P-Hydroxybenzaldehyde (2mmol), cesium carbonate (4mmol), 2-(2-methyl-5-nitro-1H-imidazol-1-yl) ethyl 4-methylbenzenesulfonate (2mmol) In a round bottom flask, add N,N-dimethylformamide (5mL), heat up to 90°C and react for 12h, TLC shows that the reaction is complete, stop the reaction, add water, extract 3 times with ethyl acetate, combine the extracts, Dry over anhydrous sodium sulfate, filter and spin dry, and the residue is separated by chromatography to obtain a yellow solid powder 4-(2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethoxy ) Benzaldehyde, 73% of productive rate. 1 HNMR(d 6 -DMSO,400MHz)δ:2.51(s,3H),4.48(t,2H),4.76(t,2H),7.01(d,2H),7.84(d,2H),8.04(s,1H),9.87 (s,1H);EIMSm / z=276[M + ].
Embodiment 3
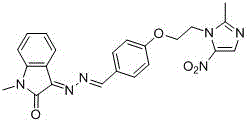
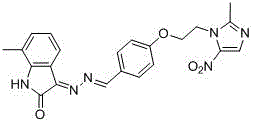
[0028] Example 3: (E)-3-((E)-(4-(2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethoxy)benzylidene)hydrazone base) preparation of indoline-2-one (1)
[0029]
[0030] Put isatin (10.00mmol) and hydrazine hydrate (11.00mmol) in a round-bottomed flask, add methanol (15.0mL), reflux for 3-8h, stop the reaction, cool to room temperature, filter the reaction solution with appropriate amount of methanol The filter cake was washed and dried to obtain 3-hydrazinoindolin-2-one as a yellow solid powder; the yield was 72%. 3-Diaminoindoline-2-one (1.00mmol), 4-(2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethoxy)benzaldehyde (1.00 mmol) in a round-bottomed flask, add ethanol (6.0mL), glacial acetic acid (1 drop), heat up to reflux, react for 4-8h, TLC shows that the reaction is complete, stop the reaction, cool to room temperature, suction filter, and use an appropriate amount of ethanol The filter cake was washed to obtain a crude product, which was purified by silica gel column chromatography to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com