Isatin imidazole compounds as well as preparation method and application thereof
An azole compound and compound technology, applied in the field of chemical synthesis, can solve problems such as the failure of antibiotics and synthetic drugs, and achieve the effect of solving drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
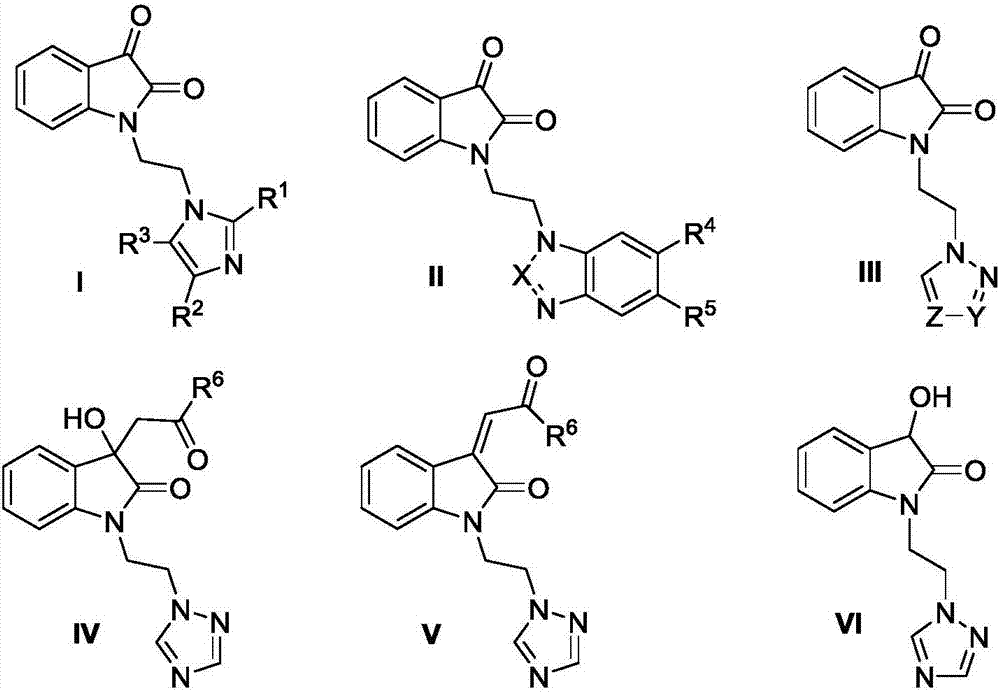
[0036] Embodiment 1, the preparation of compound VII
[0037]
[0038] Sodium hydride (1.20g, 51mmol) and isatin (5.00g, 34mmol) were added to a 250mL round-bottomed flask, and N,N-dimethylformamide (100mL) was used as solvent, stirred at 0°C for 5 minutes, slowly 1,2-Dibromoethane (4.30mL, 51mmol) was added dropwise, reacted at 60°C under nitrogen protection, followed by TLC until the reaction was completed. Ice water was added to precipitate an orange solid, which was dried and purified to obtain compound VII (6.10 g), with a yield of 70.9%.
Embodiment 2
[0039] Embodiment 2, the preparation of compound I-1
[0040]
[0041]Add 2-methyl-5-nitroimidazole (1.27g, 10mmol) and potassium carbonate (0.75g, 5.5mmol) into a 50mL round-bottomed flask, stir with 20mL acetonitrile at 60°C for 1 hour, then add isatin Derivative VII (1.27g, 5mmol), continued to stir the reaction at 60°C, followed by TLC until the end of the reaction. Acetonitrile was distilled off under reduced pressure, then extracted with water and ethyl acetate, and then concentrated, recrystallized, dried and other post-treatments to obtain compound I-1 (0.86g), with a yield of 57.3%; yellow solid; melting point: 196–198°C . 1 H NMR (600MHz, DMSO-d 6 ,ppm): δ8.40(s,1H,Im-H-4),7.61(t,J=7.7Hz,1H,isatin-H-6),7.56(d,J=7.3Hz,1H,isatin- H-4), 7.12(t, J=7.4Hz, 1H, isatin-H-5), 7.02(d, J=7.9Hz, 1H, isatin-H-7), 4.28(t, J=5.8Hz, 2H, Im-CH 2 ), 4.07(t, J=5.8Hz, 2H, Im-CH 2 CH 2 ),2.34(s,3H,Im-CH 3 ).
Embodiment 3
[0042] Embodiment 3, the preparation of compound 1-2
[0043]
[0044] Add 4-nitroimidazole (1.13g, 10mmol) and potassium carbonate (0.75g, 5.5mmol) into a 50mL round-bottomed flask, use 20mL of acetonitrile as solvent and stir at 60°C for 1 hour, then add isatin derivative VII (1.27 g, 5mmol), continue to stir the reaction at 60°C, and follow the thin layer chromatography until the end of the reaction. Acetonitrile was distilled off under reduced pressure, then extracted with water and ethyl acetate, and then concentrated, recrystallized, dried and other post-treatments to obtain compound I-2 (0.77g), with a yield of 53.8%; yellow solid; melting point: 190–192°C . 1 HNMR (600MHz, DMSO-d 6 ,ppm):δ8.54(s,1H,Im-H-5),7.90(s,1H,Im-H-3),7.57-7.53(m,2H,isatin-H-4,isatin-H- 6), 7.11(t, J=7.5Hz, 1H, isatin-H-5), 6.92(d, J=7.9Hz, 1H, isatin-H-7), 4.35(t, J=5.5Hz, 2H, Im-CH 2 ), 4.10(t, J=5.5Hz, 2H, Im-CH 2 CH 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com