Cardo structure-containing isatin aromatic hydrocarbon copolymer, preparation method and application thereof
A technology of isatin aromatics and copolymers, which is applied in the field of polymer materials and polymer ion exchange membrane preparation, can solve the problems of low battery performance, poor mechanical properties and chemical stability, etc., and achieve good mechanical properties and stable alkali resistance High performance, the effect of improving battery performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
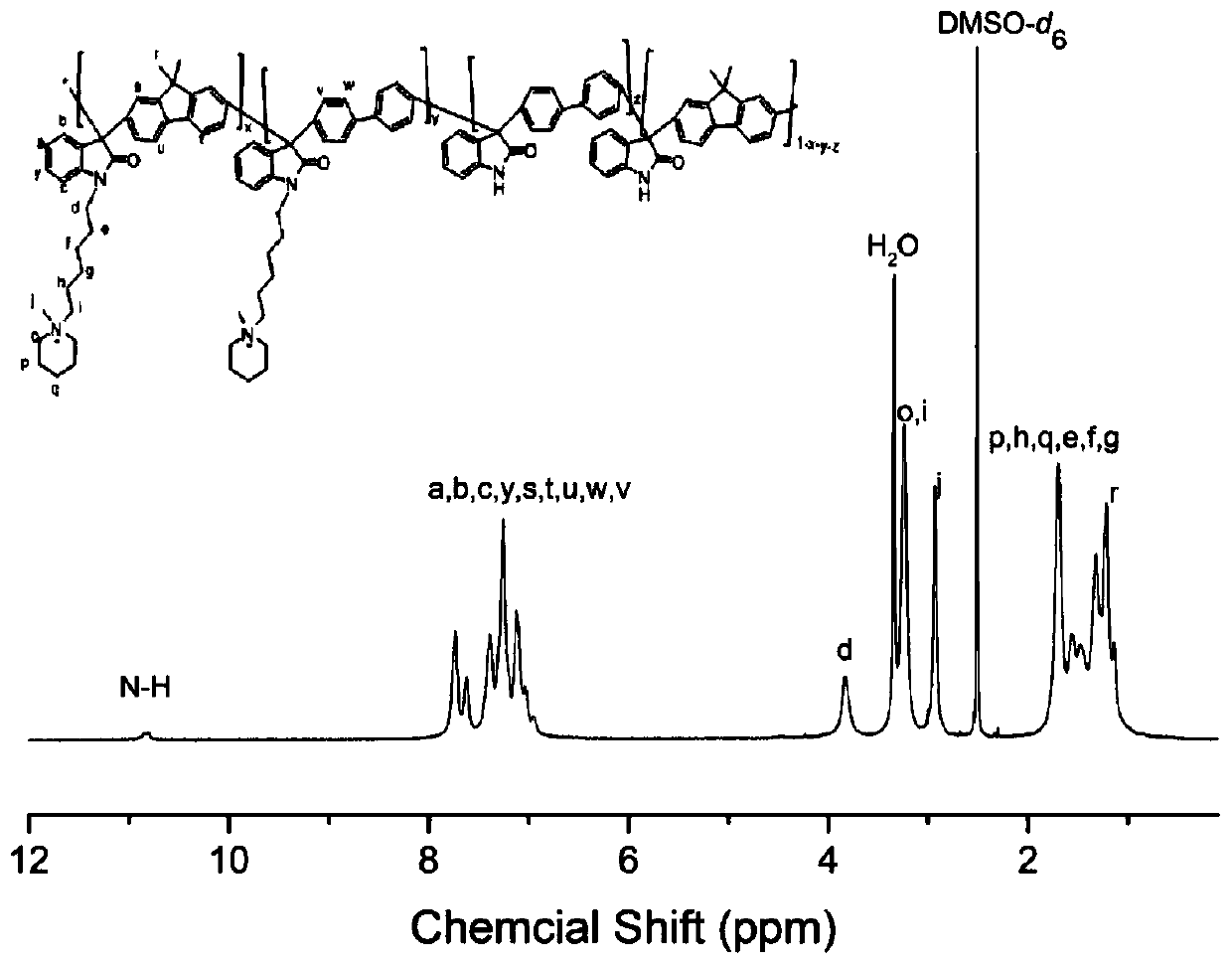
[0081] Synthesis of bromo N-methylhexylpiperidine salt: Dissolve 2mol of 1,6-dibromohexane in acetone to make a 20% solution, add 1mol of N-methylpiperidine, and react at 40°C for 24h. After centrifugation, the product was washed with acetone several times and dried to obtain bromo N-methylhexylpiperidine salt.
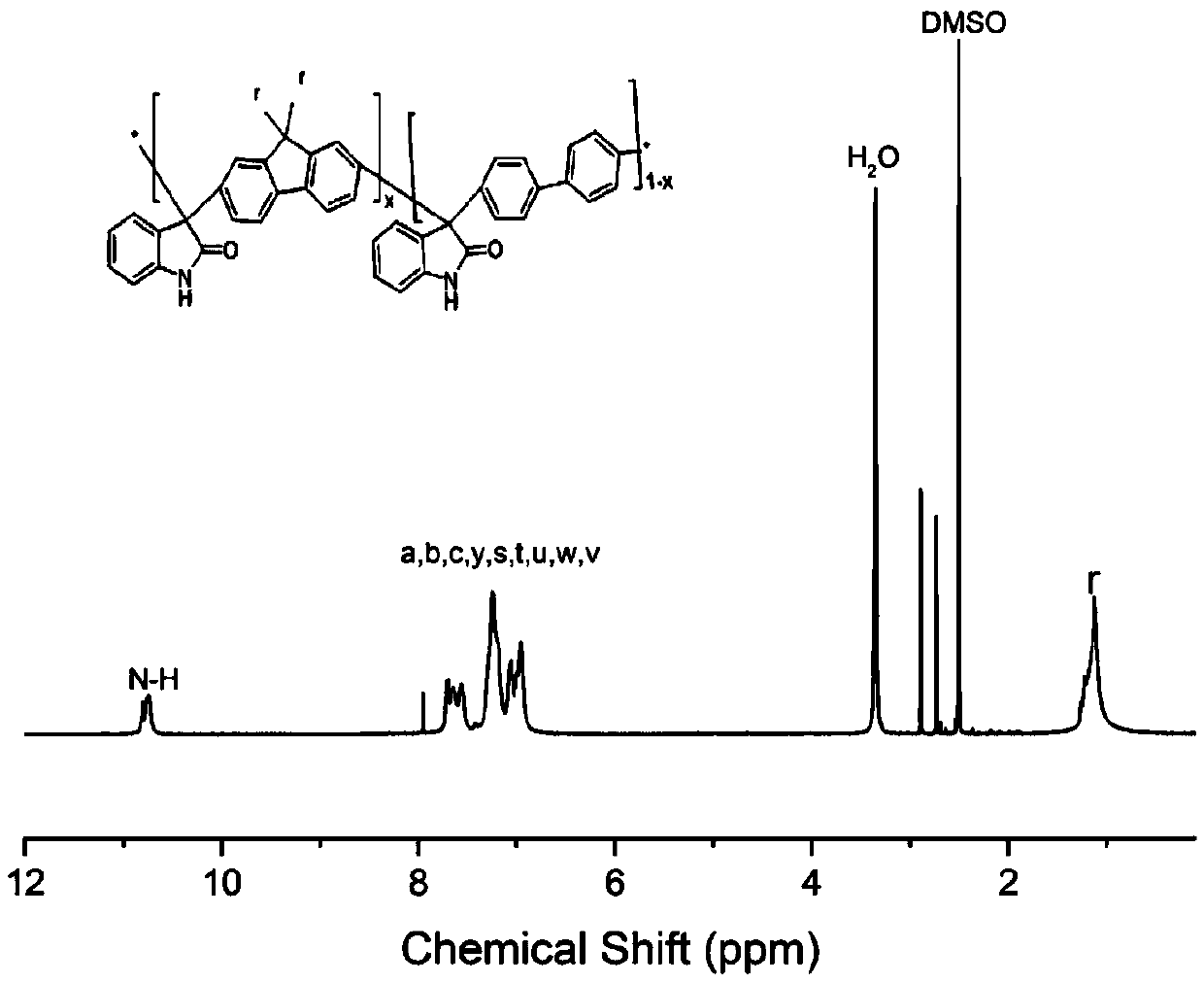
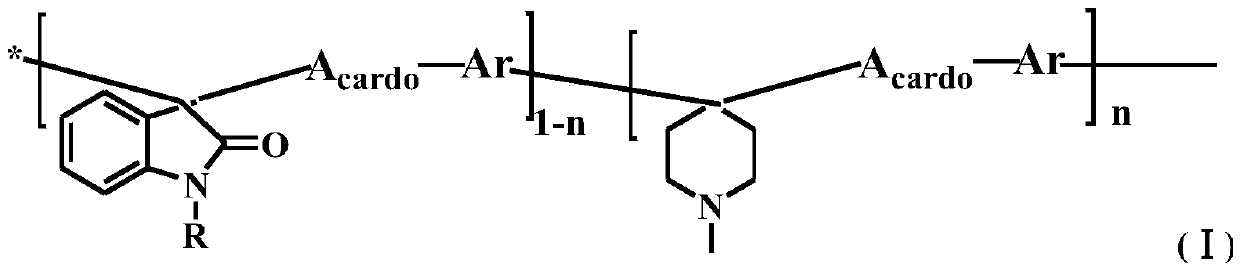
[0082] Add 9,9-dimethylfluorene, isatin and biphenyl into the reactor and stir at 0°C, add trifluoroacetic acid and the catalyst trifluoromethanesulfonic acid, so that the total monomer concentration is 30wt%. After reacting at 15°C for 50h, the product is a homogeneous viscous liquid. At the end of the reaction, pour the reaction solution into a large amount of deionized water, and put the precipitated polymer into 1M NaHCO 3 Excess acid is removed by stirring, washed with water, filtered, and dried in vacuum to obtain isatin-fluorene copolymer. Among them, the molar ratio of 9,9-dimethylfluorene to biphenyl is 6:4; the ratio of the total moles of 9,9-dimethylfluorene ...
Embodiment 2
[0094] The method for the synthesis of bromo N-methylhexylpyrrole salt is the same as in Example 1, wherein the volume concentration of 1,6-dibromohexane when dissolved in the solvent is 10%. The molar ratio of -methylpyrrole is 3:1, and the reaction is carried out at 10°C for 60h.
[0095] 1mmol of fluorene, 8mmol of bromohexane, 0.75ml of 50% NaOH aqueous solution and a small amount of tetrabutylammonium iodide were added to the reaction flask, and the refrigeration-circulation pumping was repeated three times and reacted at 80°C for 10h. Then it was purified with a silica gel column using n-hexane as the eluent to obtain 9,9-dihexylfluorene.
[0096] Add 9,9-dihexylfluorene, isatin and p-terphenyl into the reactor and stir at 0°C, add trifluoroacetic acid and the catalyst trifluoromethanesulfonic acid, so that the total monomer concentration is 10wt%. After reacting for 45 hours at 0°C, the product is a dark green uniform viscous liquid. At the end of the reaction, pour the vi...
Embodiment 3
[0103] The method for the synthesis of bromo N-methylhexylpiperidine salt is the same as in Example 1, wherein the volume concentration of 1,6-dibromohexane when dissolved in a solvent is 40%. The molar ratio of N-methylpiperidine was 10:1, and the reaction was conducted at 60°C for 48 hours.
[0104] Add 9,9-dimethylfluorene, isatin and p-terphenyl into the reactor and stir at 0°C, add trifluoroacetic acid and the catalyst trifluoromethanesulfonic acid, so that the total monomer concentration is 20wt%. After reacting at 5°C for 48h, the product is a dark green uniform viscous liquid. At the end of the reaction, pour the viscous reaction liquid into a large amount of deionized water, and put the precipitated polymer into 1M NaHCO 3 Excessive acid was removed by stirring in the mixture, washed with water, filtered, and dried in vacuum to obtain a copolymer of isatin dimethyl fluorene. Among them, the molar ratio of 9,9-dimethylfluorene to p-terphenyl is 8:2; the ratio of the tota...
PUM
| Property | Measurement | Unit |
|---|---|---|
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com