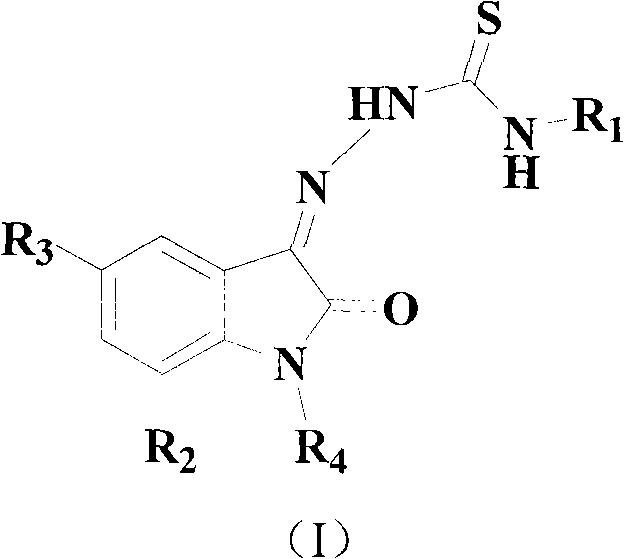
Isatin derivative and application thereof in preparation of medicines for resisting super-drug-resistance bacteria
A technology of indolindione and antibacterial drugs, which is applied in the field of indolindione derivatives and the preparation of anti-drug-resistant bacteria drugs, can solve the problems of not indicating the inhibitory effect of drug-resistant bacteria, and achieve a strong inhibitory effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: the synthesis of compound WJG-1
[0030]
[0031] Add 3.0g (20mmol) indolindione into 40mL glacial acetic acid, add 9.8g Br dropwise at room temperature 2 (61mmol) is dissolved in the solution of 10mL acetic acid, stirs 0.5h, then heats up and refluxes 1.5h, separates out red crystal, suction filtration, oven dry, obtain product 5,7-dibromoindolindione 5.8g, productive rate is about 94%.
[0032]Add 2.0g (6.6mmol) of 5,7-dibromoindolindione and 0.9g (6.6mmol) of anhydrous potassium carbonate into a 100mL three-necked flask filled with 45mL of DMF, heat to 65°C, and add 1.9g after 1h (13.2mmol) m-fluorine substituted benzyl chloride, after 2 hours of reaction, 2 times the volume of water was added, filtered and washed with water to obtain a red solid, and dried to obtain N-m-fluorobenzyl-5,7-dibromoindolindione 2.4 g, yield 89%.
[0033] Add 6.17g (37mmol) of 30% hydrazine hydrate into the reaction flask, acidify with 35% hydrochloric acid under cooli...
Embodiment 2
[0036] Embodiment 2: the synthesis of compound WJG-4
[0037]
[0038] Add 4.0mL (40mmol) of chloral hydrate and 45.9g (324mmol) of anhydrous sodium sulfate to 120mL of water. After the internal temperature rises to 40°C, stir until it becomes clear. Add 7.9g (36.0mmol) of o-iodine dropwise within 10 to 15 minutes. A solution composed of aniline and 50mL 5% hydrochloric acid, continue to react at this temperature for 0.5h, a large amount of white precipitates precipitate, quickly add 7.5g (108mmol) of hydroxylamine hydrochloride and raise the internal temperature to 75°C, a flocculent solid is formed, continue to react for 4h After that, the reaction was stopped, cooled to room temperature, filtered and dried to obtain 8.9 g of 2-iodo-1-isonitroso-acetanilide brown powder with a yield of 85%.
[0039] Preheat 25mL of concentrated sulfuric acid to 75°C, control the reaction temperature at 75-80°C, add 7.3g (25mmol) 2-iodo-1-isonitroso-acetanilide in batches within 0.5h under...
Embodiment 3
[0044] Example 3: Determination of anti-MRSA activity of indolindione derivatives.
[0045] 1. Test material
[0046] Mueller-Hinton Broth (Beijing Oboxing Biotechnology Co., Ltd.), tryptone (UK OXOID company), yeast extract powder (UK OXOID company), sodium chloride (Sinopharm Chemical Reagent Co., Ltd.), 96-well cell culture plate ( flat bottom) (Corning, U.S.), positive control drug vancomycin (Amresco, U.S.), dimethyl sulfoxide DMSO (Sinopharm Chemical Reagent Co., Ltd.), MHB medium (use an electronic balance to weigh 24 grams of Mueller-HintonBroth The dry powder was dissolved in 1000 ml of distilled water, adjusted to pH 7.2, and sterilized at 121° C. for 20 minutes using an autoclave). Methicillin-resistant Staphylococcus aureus MRSA (clinically isolated drug-resistant strain from Beijing Chaoyang Hospital Affiliated to Capital Medical University, stored in a freezer at -80°C in glycerol tubes), Staphylococcus aureus (Staphylococcusaureus ATCC6538), Bacillus subtilis (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com