Dihydroisoquinoline compounds and application of dihydroisoquinoline compounds for preparing antibacterial agents for plants
A dihydroisoquinoline and plant antibacterial technology, which is applied in the application field of preparing plant antibacterial drugs, can solve problems such as research reports on the biological activity of compounds that have not been seen, and achieve the effects of low production cost, simple structure, and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
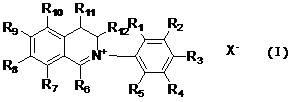
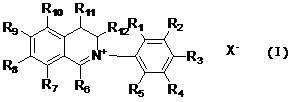
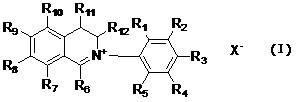
[0013] A class of dihydroisoquinoline compounds involved in the present invention and their application as preparation of plant antibacterial drugs are characterized in that they have the following molecular structure characteristics:
[0014]
[0015] Among them, R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 , R 9 , R 10 , R 11 , R 12 are the same or different and represent hydrogen, alkyl, cycloalkyl, alkenyl, alkynyl, unsaturated monocyclic hydrocarbon, alkoxy, halogen, hydroxyl, nitro, trifluoromethyl, heterocyclic substitution group, carboxyl group, ester group, amido group, acyl group, aldehyde group.
[0016] x - It is sulfate, halogen anion, carbonate, bicarbonate, phosphate, hydrogen phosphate, fatty acid acid, sulfonate.
[0017] As the application of dihydroisoquinoline compounds in the preparation of plant antibacterial drugs, it is known that dihydroisoquinoline compounds have significant inhibitory activity on the following plant pathogens: Curvul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com