Spiro-heterocyclic compound containing indole structures and preparation method of spiro-heterocyclic compound
A compound and catalyst technology, applied in the field of spiro heterocyclic compounds and their preparation, can solve the problems of cumbersome processing, and achieve the effects of simple post-processing, high yield, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
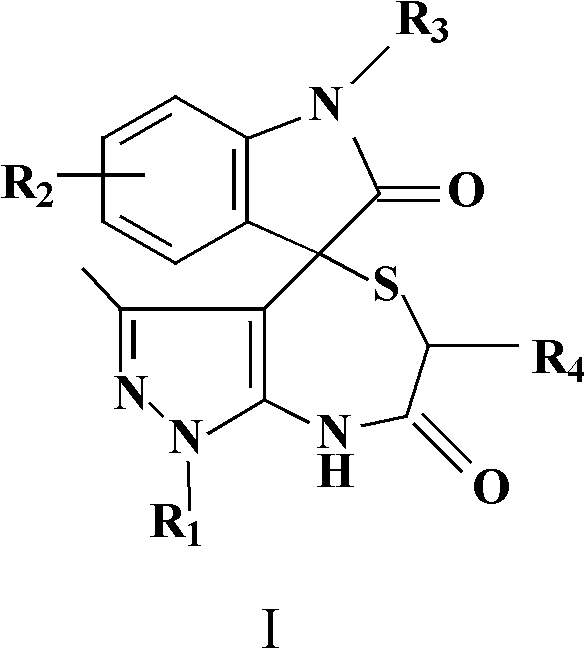
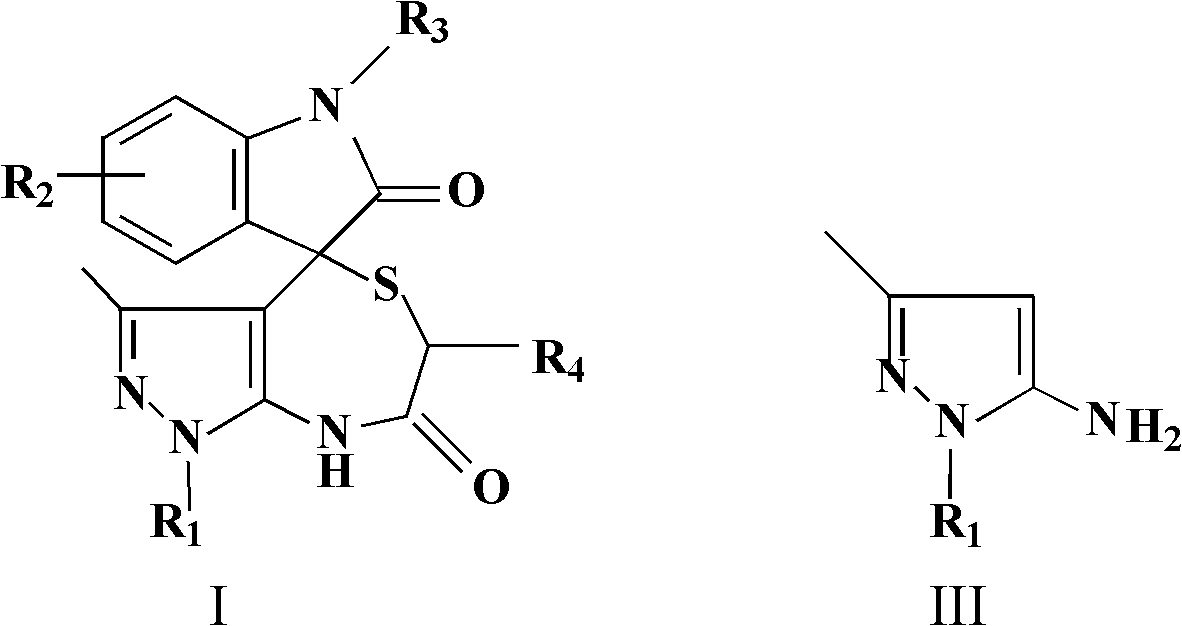
[0046] Isatin (1mmol, 0.147g), 1-phenyl-5-aminopyrazole (1mmol, 0.173g) and thioglycolic acid (1mmol, 0.092g) were added together in a 50mL short-necked round bottom flask, and 5mL of acetonitrile was added, and Add 0.057g of p-toluenesulfonic acid after adding 0.057g of p-toluenesulfonic acid, add a reflux condenser to reflux for 24 hours, monitor the reaction progress with TLC, spin the reaction system to dry after the reaction is completed, wash with absolute ethanol, and suction filter. Drying gave a white solid as 3'-methyl-1'-phenyl-6',8'-dihydrospiro[indole-3,4'-pyrazolo[3,4-e][1,4 ]thiazepine]-2,7'(1'H)-dione 0.32 g, yield 86%. As determined by nuclear magnetic resonance spectroscopy, the melting point is 256-257°C; 1 H NMR (300MHz, DMSO-d 6 ):δ H 1.46(s, 3H, CH 3 ), 3.11 (d, J=15.0Hz, 1H, CH 2 ), 4.54 (d, J=15.0Hz, 1H, CH 2 ), 6.96-7.04(m, 2H, ArH), 7.15(d, J=7.2Hz, 1H, ArH), 7.32(t, J=7.8Hz, 1H, ArH), 7.40(t, J=6.9Hz, 1H, ArH), 7.47-7.56 (m, 4H, ArH), 9.94 (s,...
Embodiment 2
[0048] Isatin (2mmol, 0.294g), 1-methyl-5-aminopyrazole (1mmol, 0.111g) and 2-mercaptopropionic acid (1.5mmol, 0.159g) were added together in a 50mL short-necked round bottom flask, and 5mL of ethanol, shake slightly to mix the substrate evenly, add 0.001g of p-toluenesulfonic acid, add a reflux condenser to reflux for 8h, monitor the reaction progress with TLC, spin the reaction system to dry after the reaction is completed, and wash with absolute ethanol , suction filtration, and drying to obtain a white solid, 1',3',6'-trimethyl-6',8'-dihydrospiro[indole-3,4'-pyrazolo[3,4-e ][1,4]Thiazepine]-2,7'(1'H)-dione 0.27g, yield 83%. As determined by nuclear magnetic resonance spectroscopy, the melting point is 181-183°C; 1 H NMR (400MHz, DMSO-d 6 ):δ H 1.24(d, J=6.8Hz, 3H, CH 3 ), 1.38 (s, 3H, CH 3 ), 3.66(s, 3H, CH 3 ), 4.53(q, J=7.2Hz, 1H, CH), 6.94-7.00(m, 2H, ArH), 7.05(d, 1H, J=7.2Hz, ArH), 7.29(t, 1H, J=7.6 Hz, ArH), 10.11(s, 1H, NH), 10.75(s, 1H, NH). 13 C NMR (75MHz...
Embodiment 3-20
[0050] According to the method of embodiment 1 and embodiment 2, isatin is replaced with 5-methyl isatin, 5-bromoisatin, 6-bromoisatin, 5-chloroisatin, 5-fluoroisatin and 1-methyl isatin Any one of isatins, the aminopyrazole is 1-phenyl-5-aminopyrazole or 1-methyl-5-aminopyrazole, and the mercaptocarboxylic acid is thioglycolic acid or 2-mercaptopropionic acid. Reflux for 8-24 hours, wash with absolute ethanol, filter with suction, and dry to obtain a solid product. The results of nuclear magnetic resonance spectroscopy are as follows:
[0051] 3',6'-Dimethyl-1'-phenyl-6',8'-dihydrospiro[indole-3,4'-pyrazolo[3,4-e][1,4]sulfur Azepine]-2,7'(1'H)-dione, yield 83%, white solid. Melting point 270-271°C; 1 H NMR (400MHz, DMSO-d 6 ):δ H 1.27(d, J=7.2Hz, 3H, CH 3 ), 1.49 (s, 3H, CH 3 ), 4.73(q, J=7.2Hz, 1H, CH), 6.98-7.03(m, 2H, ArH), 7.11(d, J=7.6Hz, 1H, ArH), 7.33(t, J=7.6Hz, 1H, ArH), 7.41(t, J=7.2Hz, 1H, ArH), 7.51(t, J=7.6Hz, 2H, ArH), 7.58(d, J=7.6Hz, 2H, ArH), 9.98(s ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com