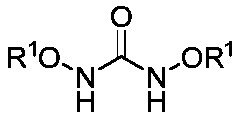
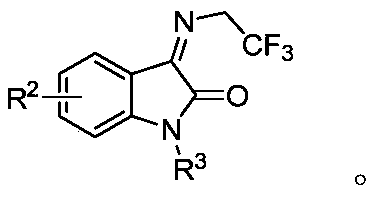
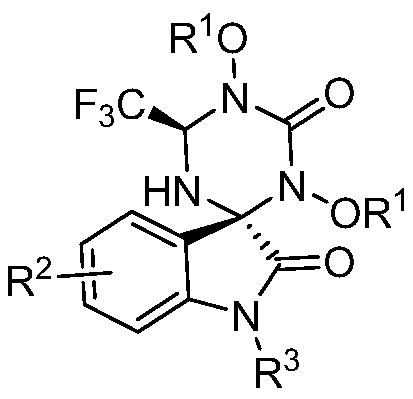
1, 3, 5-triazine-2-ketospirooxindole compound and preparation method
A technology for spirocyclic indolinone and compound is applied in the field of compound preparation and achieves the effects of simple operation, easy preparation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
[0031] Weigh N, N'-dialkoxy substituted urea 1a (27.2 mg, 0.1 mmol) and trifluoroethyl substituted isatin-3-imine 2a (48.4 mg, 0.2 mmol) and dissolve in DME (0.5 mL ), which was slowly added to a solution of NaH (12.0 mg, 0.3 mmol, 60% dispersed in kerosene) in TFP (0.5 mL) at 0°C. Then weigh the oxidant PhI(OH)(OTs) (78.4mg, 0.2mmol), dissolve it in DME (1.5mL), and slowly add it dropwise to the reaction system within one minute, and react at 0°C to room temperature. The mixed solution was stirred well (the reaction was checked by TLC) until the trifluoroethyl-substituted isatin-3-imine 2a was completely consumed. Finally, the reaction mixture solution was concentrated under reduced pressure, and the crude product was subjected to column chromatography (the eluent was selected as a mixed solution of petroleum ether / ethyl acetate with a volume ratio of 5:1) to obtain the target product trans-3aa (33.3 mg), The yield was 65%.
[0032] Characterization and analysis...
Embodiment 2
[0034]
[0035]Weigh N, N'-dialkoxy substituted urea 1a (27.2 mg, 0.1 mmol) and trifluoroethyl substituted isatin-3-imine 2b (64.0 mg, 0.2 mmol) and dissolve in DME (0.5 mL ) was slowly added to a solution of NaH (12.0 mg, 0.3 mmol, 60% dispersed in kerosene) in TFP (0.5 mL) at 0°C. Then weigh the oxidant PhI(OH)(OTs) (78.4mg, 0.2mmol), dissolve it in DME (1.5mL), and slowly add it dropwise to the reaction system within one minute, and react at 0°C to room temperature. The mixed solution was stirred well (the reaction was monitored by TLC) until the trifluoroethyl-substituted isatin-3-imine 2b was completely consumed. Finally, the reaction mixture solution was concentrated under reduced pressure, and the crude product was subjected to column chromatography (the eluent was selected as a mixed solution of petroleum ether / ethyl acetate with a volume ratio of 5:1) to obtain the target product trans-3ab (47.2 mg), The yield was 80%.
[0036] Characterization and analysis of th...
Embodiment 3
[0038]
[0039] Weigh N,N'-dialkoxy substituted urea 1a (27.2 mg, 0.1 mmol) and trifluoroethyl substituted isatin-3-imine 2c (55.2 mg, 0.2 mmol) and dissolve in DME (0.5 mL ) was slowly added to a solution of NaH (12.0 mg, 0.3 mmol, 60% dispersed in kerosene) in TFP (0.5 mL) at 0°C. Then weigh the oxidant PhI(OH)(OTs) (78.4mg, 0.2mmol), dissolve it in DME (1.5mL), and slowly add it dropwise to the reaction system within one minute, and react at 0°C to room temperature. The mixed solution was stirred well (the reaction was checked by TLC) until the trifluoroethyl-substituted isatin-3-imine 2c was completely consumed. Finally, the reaction mixture solution was concentrated under reduced pressure, and the crude product was subjected to column chromatography (the eluent was selected as a mixed solution of petroleum ether / ethyl acetate with a volume ratio of 5:1) to obtain the target product trans-3ac (37.1 mg), The yield was 68%.
[0040] Characterization and analysis of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com