Chiral indolopyrrole alkaloid and preparation method thereof
A technology for indolopyrrole and alkaloids, which is applied in the field of chiral indolopyrrole alkaloids and their preparation, can solve problems such as difficult multi-functional groups and limited substrate range diversity, and achieve good enantioselectivity, The effect of enriching drug molecule library and simple reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
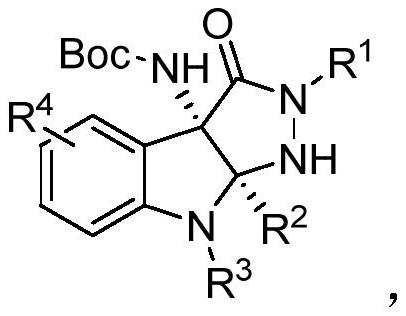
[0033] Preparation of Compound 1
[0034]
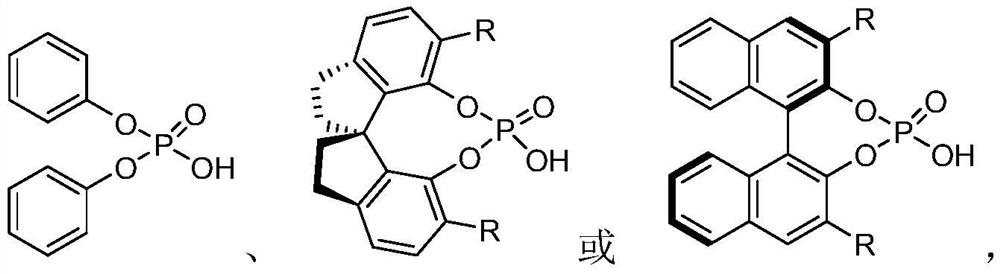
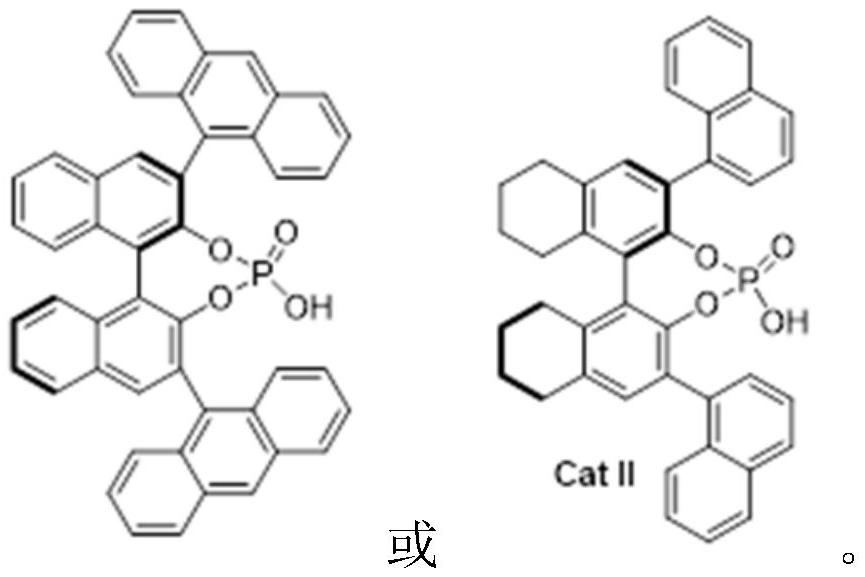
[0035] Add 0.10mmol of diphenylamine S1-1, 0.11mmol of pyrazolone ketimine S2, 0.001mmol of chiral phosphoric acid catalyst CPA I, 50mg of molecular sieves, and 1.0mL of chloroform into the reaction flask, and react at 0°C for 12h. Column separation gave white solid 1 with a yield of 92%.
Embodiment 2
[0037] Preparation of compound 2
[0038]
[0039] Add 0.10mmol 4-phenyldiphenylamine S1-2, 0.11mmol pyrazolone imine S2, 0.001mmol chiral phosphoric acid catalyst CPA I, 50mg molecular sieves, and 1.0mL chloroform into the reaction flask, react at 0°C for 12h, and the reaction is complete Afterwards, a yellow solid 2 was obtained by separation on a silica gel column with a yield of 90%.
[0040] Compound 3-15 was prepared from the corresponding aniline and pyrazolone imine according to the above method.
Embodiment 3
[0042] Preparation of compound 16
[0043]
[0044] Add 0.10mmol nitrogen phenyl 2-naphthylamine S1-16, 0.11mmol pyrazolone imine S2, 0.001mmol chiral phosphoric acid catalyst CPA II, 1.0mL DCM into the reaction flask, react at -20°C for 12h, and the reaction is complete Afterwards, a white solid 16 was obtained by separation on a silica gel column with a yield of 98%.
[0045] Compounds 17-25 were prepared from the corresponding naphthylamine and pyrazolone imine according to the above method.
[0046] min. 1 H NMR (500MHz, acetone-d 6 ):δ8.02(d,J=10.0Hz,2H)7.58(d,J=10.0Hz,2H),7.49-7.45(m,3H),7.39-7.30(m,3H),7.21(s,1H ),7.13-7.07(m,2H),6.75(t,J=10.0Hz,1H),6.44(d,J=10.0Hz,1H),5.70(s,1H),1.43(s,9H),1.40 (s,3H). 13 C NMR (126MHz, DMSO-d 6 ):δ169.43,157.17,148.62,140.76,140.49,130.81,130.00,129.40,128.09,127.09,125.96,124.65,124.54,119.30,118.71,108.05,89.52,80.61,72.55,28.41,17.63.HRMS(ESI):m / z calculated for C 27 h 28 N 4 o 3 [M+H + ]457.2234, found 457.2233. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com