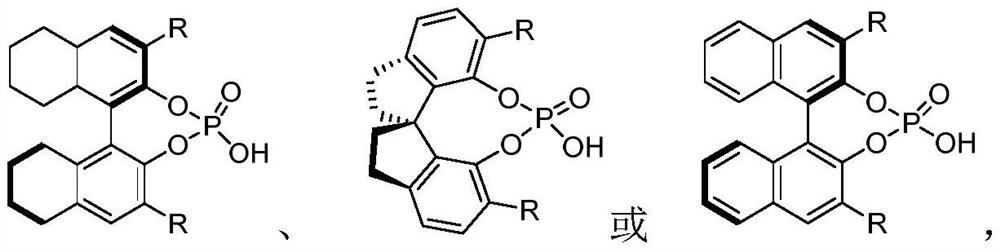
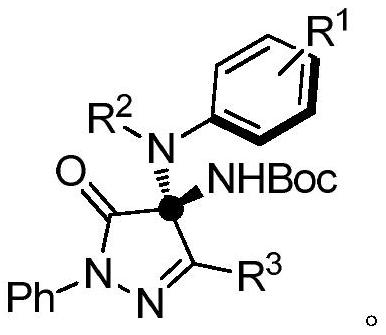
Preparation method of chiral pyrazolone compound
A technology of pyrazolone and pyrazolone imine, which is applied in the directions of organic chemistry methods, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc. Regioselectivity, increasing synthesis steps, etc., to achieve the effect of simple reaction conditions, simplified steps, and small dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of compound 3
[0034]
[0035] Chiral phosphoric acid (0.7mg, 1mol%), nitrogen methyl aniline S1-1 (10.7mg, 0.1mmol, 1.0equiv.), pyrazolone imine S-2 (31.6mg, 0.11mmol, 1.1equiv. ) placed in a dry reaction tube, reacted at room temperature, and monitored with a thin-layer chromatographic plate until the end of the reaction. The reaction solution was spin-dried and purified by column chromatography (PE:EA=20:1) to obtain compound 3 with a yield of 96%.
[0036] Yellow solid.37.8mg,96%yield.96%ee.[α] 20 D +92.71(c 1,CHCl 3 ).m.p.99-101℃.HPLC (Daicel Chiralpak AD-H, hexane / i-PrOH=80:20, 1.0mL / min, 254nm):t R (major)=4.9min,t R (minor) = 7.5min. 1 H NMR (400MHz, Acetone-d 6 ): δ7.83(d, J=8.0Hz, 2H), 7.39(t, J=8.0Hz, 2H), 7.25-7.14(m, 6H), 2.98(s, 3H), 1.89(s, 3H) ,1.33(s,9H). 13 CNMR (100MHz, Acetone-d 6 )δ168.95, 147.07, 138.50, 128.80, 128.50, 127.15, 126.75, 124.24, 118.01, 79.76, 77.59, 36.13, 27.38, 12.95. HRMS (ESI) m / z: [M+Na] + calculated ...
Embodiment 2
[0038] Embodiment 2, the preparation of compound 4
[0039] Chiral phosphoric acid (0.7mg, 1mol%), 4-methylnitromethylaniline S1-2 (12.1mg, 0.1mmol, 1.0equiv.), pyrazolone ketimine S2 (31.6mg, 0.11mmol, 1.1 equiv.) placed in a dry reaction tube, reacted at room temperature, and monitored by a thin-layer chromatographic plate until the end of the reaction. The reaction solution was spin-dried and purified by column chromatography (PE:EA=20:1) to obtain compound 4.
[0040] NMR (400MHz, Acetone-d 6 ):δ7.84(d,J=8.0Hz,2H),7.41-7.37(m,2H),7.18-7.11(m,3H),7.04(d,J=8.0Hz,2H),2.94(s, 3H), 2, 24(s, 3H), 1.89(s, 3H), 1.33(s, 9H). 13 C NMR (100MHz, Acetone-d 6)δ169.96, 157.85, 154.52, 154.32, 145.42, 139.51, 137.50, 130.30, 129.45, 128.03, 125.15, 118.96, 118.82, 80.53, 78.56, 37.16, 28.31, 20.90. Na] + calculated for C 23 h 28 N 4 o 3 Na 431.2059,found 431.2071.
example 3
[0041] Example 3, the preparation of compound 5
[0042] Chiral phosphoric acid (0.7mg, 1mol%), 4-methoxynitromethylaniline S1-3 (13.7mg, 0.1mmol, 1.0equiv.), pyrazolone imine S2 (31.6mg, 0.11mmol, 1.1 equiv.) placed in a dry reaction tube, reacted at room temperature, and monitored by thin-layer chromatography until the end of the reaction. The reaction solution was spin-dried and purified by column chromatography (PE:EA=20:1) to obtain compound 5.
[0043] (m,2H),7.17-7.15(m,3H),6.77(d,J=8.0Hz,2H),3.71(s,3H),2.92(s,3H),1.89(s,3H),1.33( s,9H). 13 C NMR (100MHz, Acetone-d 6 )δ169.96,159.41,158.00,154.39,140.52,139.50,129.44,129.40,125.13,118.94,115.43,114.75,113.72,80.64,78.64,55.59,37.29+,28.341,13. Na] + calculated for C 23 h 28 N 4 o 4 Na447.2008, found447.2019.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com