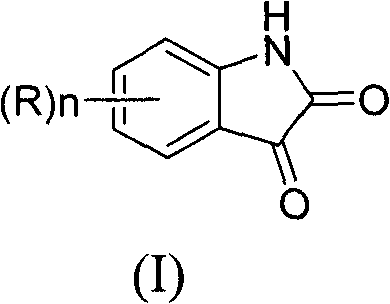
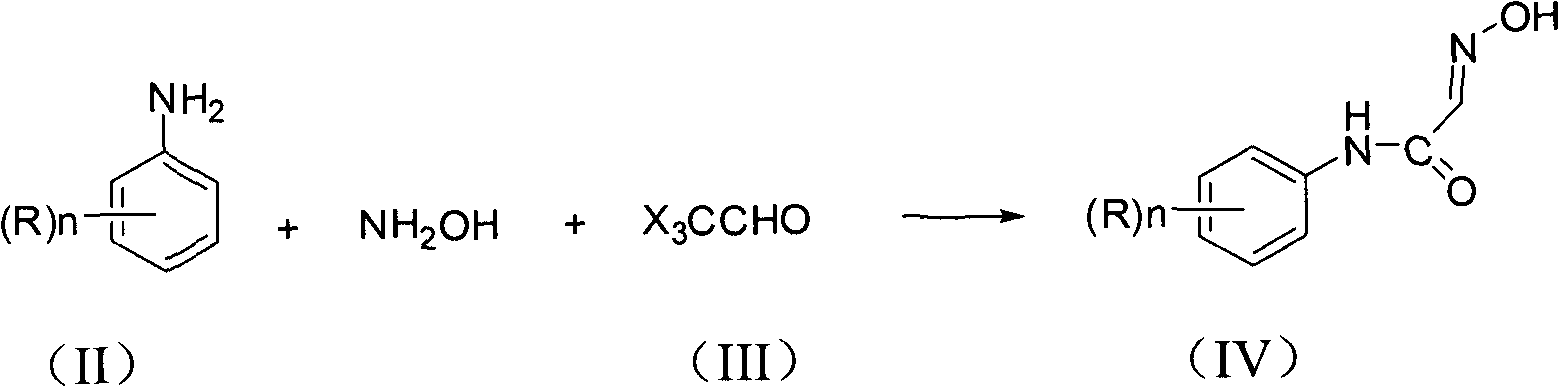Synthesis method of isatin derivatives
A synthesis method and a derivative technology are applied in the synthesis field of isatin derivatives, can solve problems such as being unsuitable for industrial production, and achieve the effects of low cost, simple process flow and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: Preparation of 7-fluoroisatin
[0036] In a 500ml four-neck flask, add 380.0g of water and 27.0g of chloral hydrate, stir and dissolve at room temperature, and then add 95.0g of anhydrous Na 2 SO 4 , heating up. The temperature was raised to 40°C, 23.0g of hydroxylamine hydrochloride, 32.0g of 30% HCl and 11.0g of 2-fluoroaniline were added, and the temperature was raised to 65°C with stirring. React at 65℃~73℃, the color of the system gradually becomes darker, Na 2 SO 4 gradually dissolved completely. After 1 hour, the reaction reached the end point, and the unreacted o-fluoroaniline was 2.6 Area%, so the reaction was stopped. Filter at 40-45°C, wash with water, and dry to obtain 17.2 g of a light brown solid with an HPLC content of 96.5%.
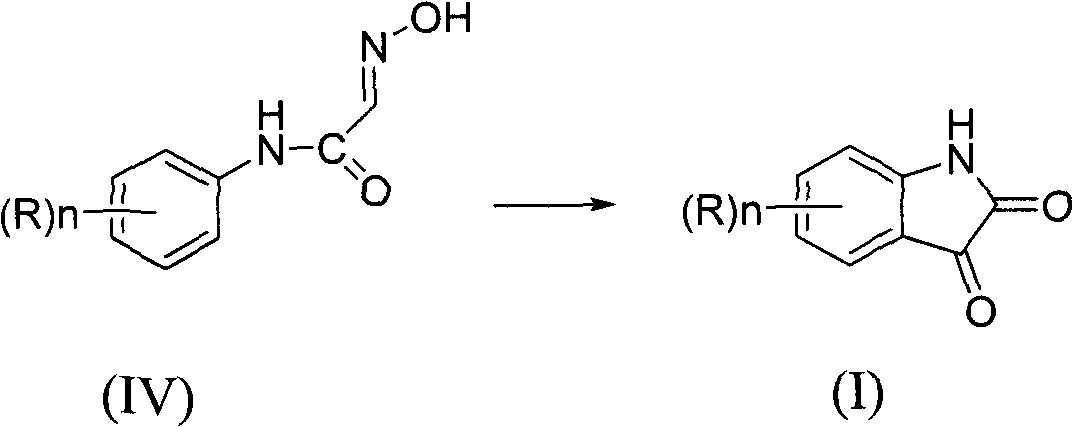
[0037] Put 50.0g polyphosphoric acid and 17.2g N-(2-fluorophenyl)-2-(hydroxyimino)acetamide into a 250ml four-necked bottle, stir, raise the temperature to 70-75°C, and maintain it at 70-75°C After 2 hours of r...
Embodiment 2
[0038] Embodiment 2: the preparation of 6-fluoroisatin
[0039] Add 71.6g of 3-fluoroaniline, 160.0g of hydroxylammonium hydrochloride and 350.0g of water into a 500ml four-necked flask, and stir at 60°C for 0.5 hours. Add 900.0g water, 120.0g chloral hydrate, 609.6g anhydrous Na to another 2L four-necked bottle 2 SO 4 , 80.0g of 30% HCl, stirred and heated to 50-55°C. After stirring at 50-55°C for 1 hour, slowly add the prepared mixed solution of 3-fluoroaniline and hydroxylamine hydrochloride dropwise. After the dropwise addition, react at 75-80°C for 2 hours, and the reaction of 3-fluoroaniline is complete. Reduce the temperature of the kettle to 45-50°C, filter under reduced pressure, wash with water, and dry to obtain 112.9 g of a light brown solid with an HPLC content of 97.5%. The filtrate was adjusted to pH 7 with 10% NaOH, and 10% of water was distilled off for further use.
[0040] 146.0 g of polyphosphoric acid was put into a 1 L four-necked bottle, and 45.0 g ...
Embodiment 3
[0041] Embodiment 3: the preparation of 5-fluoroisatin
[0042] Add 35.8g of 4-fluoroaniline, 80.0g of hydroxylammonium hydrochloride and 100.0g of water into a 250ml four-necked flask, and stir at 60°C for 0.5 hours. Add 400.0g water, 60.0g chloral hydrate, 200.0g anhydrous Na to another 1L four-necked bottle 2 SO 4 , 40.0g of 30% HCl, stirred and heated to 50-55°C. After stirring at 50-55°C for 1 hour, slowly add the prepared mixed solution of 4-fluoroaniline and hydroxylamine hydrochloride dropwise. After the dropwise addition, react at 75-80°C for 2 hours, and the reaction of 4-fluoroaniline is complete. Reduce the temperature of the kettle to 45-50°C, filter under reduced pressure, wash with water, and dry to obtain 56.2 g of a light brown solid with an HPLC content of 95.8%. The filtrate was adjusted to pH 7 with 10% NaOH, and 10% of water was distilled off for further use.
[0043] Put 50.0ml of boron trifluoride / tetrahydrofuran, 36.4g of N-(4-fluorophenyl)-2-(hydr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com