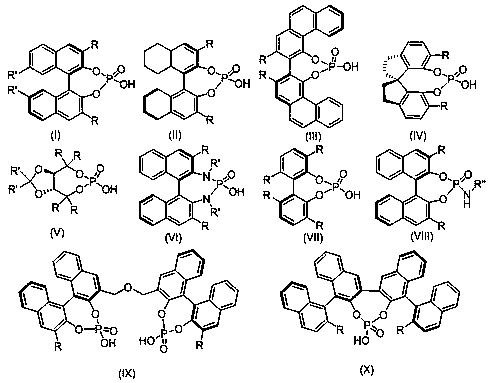
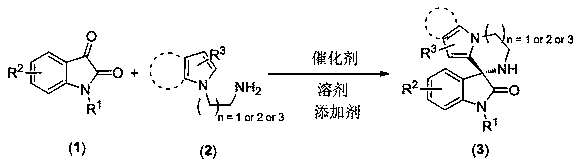
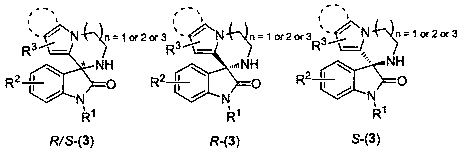
Pyrrolo or indolo azacycloalkane structure containing chiral spiro oxyindole compound as well as racemate and preparation method thereof
A technology of azacycloalkanes and compounds is applied in the fields of chiral oxospirocyclic indole compounds and their racemates and preparations, and can solve the problems of achiral synthesis, high price of rhodium, low reaction yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: (-)-1-Benzyl-3',4'-dihydro-2'H-spiro[indoline-3,1'-pyrrolo[1,2-a]pyrazine] Synthesis of -2-one:
[0054] The structural formula of this example is as follows:
[0055]
[0056] To 2-(1H-pyrrol-1-yl)ethylamine (0.24mmol) and N-benzyl isatin (0.2mmol) in dichloromethane solution (2mL), add chiral phosphoric acid catalyst IV-12 (10mol% )and Molecular sieves can be reacted overnight at 25°C. The reaction solution was directly purified by column chromatography to obtain the target product (-)-1-benzyl-3',4'-dihydro-2'H-spiro[indoline-3,1'-pyrrolo[1, 2-a]pyrazine]-2-one: 55.7mg, yield: 85%, enantiomeric excess value (ee value): 88%, yellow solid, [α] 25 D =–82.13 (c=0.88, CH 2 Cl 2 ), mp: 119-121°C; 1 H NMR (400MHz, CDCl 3 )δ7.38–7.30(m,4H),7.30–7.26(m,2H),7.22(td,J=7.8,1.3Hz,1H),7.03(td,J=7.5,1.0Hz,1H),6.75 (d,J=7.8Hz,1H),6.69(dd,J=2.7,1.7Hz,1H),6.13(dd,J=3.6,2.7Hz,1H),5.53(dd,J=3.6,1.6Hz, 1H), 5.04(d, J=15.7Hz, 1H), 4.77(d, J=15.7Hz, 1H), 4.30(ddd,...
Embodiment 2
[0058] Example 2: (-)-1-methyl-3',4'-dihydro-2'H-spiro[indoline-3,1'-pyrrolo[1,2-a]pyrazine] Synthesis of -2-one:
[0059] The molecular structure of this example is as follows:
[0060]
[0061] To 2-(1H-pyrrol-1-yl)ethylamine (0.24mmol) and N-methylisatin (0.2mmol) in dichloromethane solution (2mL), add chiral phosphoric acid catalyst IV-12 (10mol% )and Molecular sieves can be reacted overnight at 25°C. The reaction solution was directly purified by column chromatography to obtain the target product (-)-1-methyl-3',4'-dihydro-2'H-spiro[indoline-3,1'-pyrrolo[1, 2-a]pyrazine]-2-one, yellow solid, 34.3 mg, yield: 68%, enantiomeric excess value (ee value): 68%, [α] 25 D =–89.52 (c=0.83, CH 2 Cl 2 ), mp: 122.7-125.4°C. [α] 25 D =–89.52 (c=0.83, CH 2 Cl 2 ). 1 HNMR (400MHz, CDCl 3 )δ7.33(tt, J=7.7,1.0Hz,1H),7.24(s,1H),7.05(t,J=7.5Hz,1H),6.86(d,J=7.8Hz,1H),6.64( t,J=2.2Hz,1H),6.07(t,J=3.2Hz,1H),5.47(dd,J=3.7,1.6Hz,1H),4.20(dtd,J=11.3,8.0,7.1,4.2Hz ,1H), 4.12(dt,...
Embodiment 3
[0063] Example 3: (-)-1-isopropyl-3',4'-dihydro-2'H-spiro[indoline-3,1'-pyrrolo[1,2-a]pyrazine Synthesis of ]-2-one:
[0064] The molecular structure of this example is as follows:
[0065]
[0066] To 2-(1H-pyrrol-1-yl)ethylamine (0.24mmol) and N-isopropyl isatin (0.2mmol) in dichloromethane solution (2mL), add chiral phosphoric acid catalyst IV-12 (10mol %)and Molecular sieves can be reacted overnight at 25°C. The reaction solution was directly purified by column chromatography to obtain the target product (-)-1-isopropyl-3',4'-dihydro-2'H-spiro[indoline-3,1'-pyrrolo[1 ,2-a]pyrazine]-2-one, yellow solid, 54.2mg, yield: 97%, enantiomeric excess value (ee value): 74%, [α] 25 D =–80.15 (c=0.85, CH 2 Cl 2 ), mp: 144-147°C. [α] 25 D =–80.15 (c=0.85, CH 2 Cl 2 ). 1 H NMR (400MHz, CDCl 3 )δ7.34–7.29(m,1H),7.27(s,1H),7.05(d,J=7.8Hz,2H),6.69–6.60(m,1H),6.09(t,J=3.2Hz,1H ),5.48(dd,J=3.7,1.7Hz,1H),4.58(p,J=7.0Hz,1H),4.21(td,J=8.5,4.7Hz,1H),4.18–4.08(m,2H) ,3.30(dt,J...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com