Pyrazolecarboxylic acid copper complex and preparation method and application thereof
A technology of copper pyrazole carboxylate and copper complexes, which is applied in the directions of organic compound/hydride/coordination complex catalysts, organic chemical methods, chemical instruments and methods, etc. The effect of good selectivity, high yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: A method for preparing a copper pyrazole carboxylate complex with catalytic properties
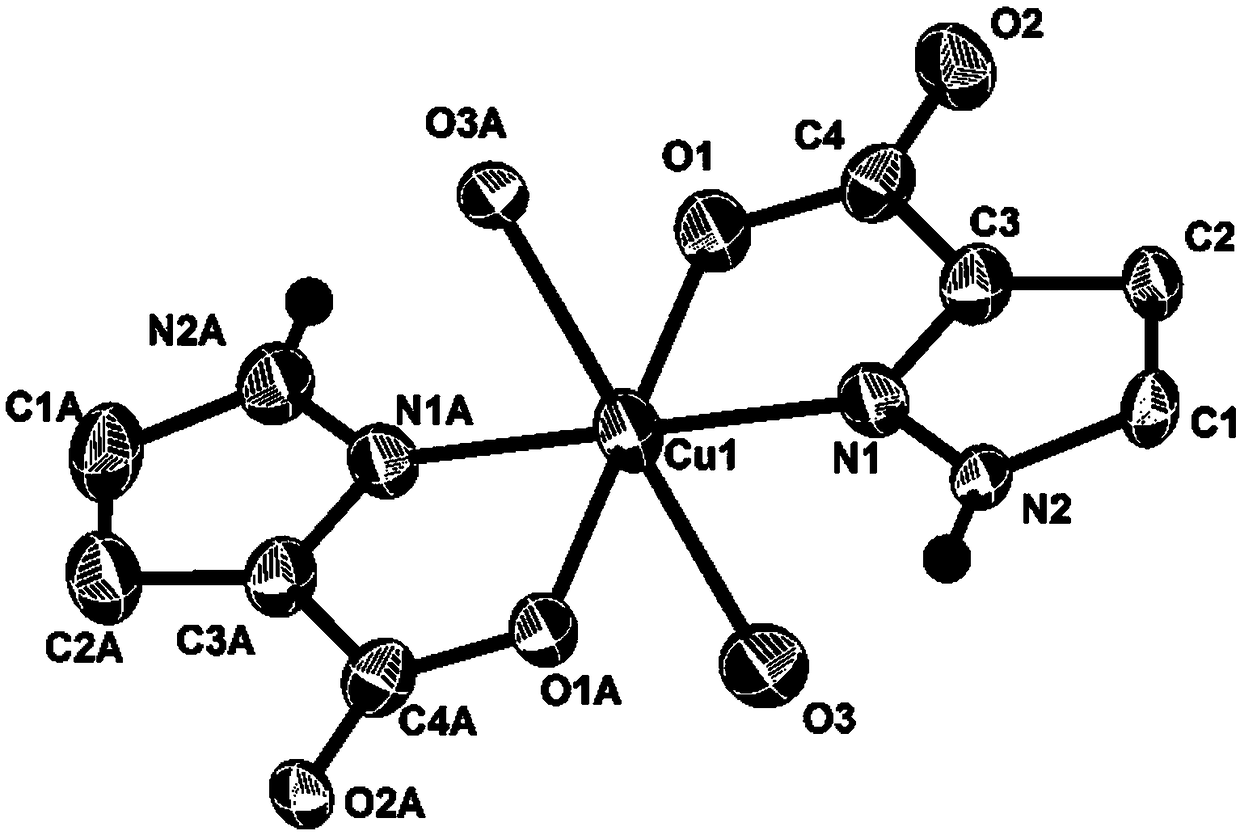
[0022] Dissolve the pyrazole-3-carboxylic acid ligand in N,N-dimethylformamide solvent, add a mixed solution of copper acetate and lanthanum chloride, mix well, react in an oven at 130°C for 2 days, and cool to room temperature to obtain Blue bulk copper complex single crystal; (the molar ratio of pyrazole-3-carboxylic acid ligand to copper acetate and lanthanum chloride is 6:1:1, the N,N-dimethylformamide solvent and distilled water The volume ratio is 1:2.5); yield: 60.7%.
Embodiment 2
[0023] Embodiment 2: A kind of preparation method of pyrazole carboxylate copper complex with catalytic performance
[0024] Dissolve the pyrazole-3-carboxylic acid ligand in N,N-dimethylformamide solvent, add a mixed solution of copper acetate and lanthanum chloride, mix well, react in an oven at 130°C for 4 days, and cool to room temperature to obtain Blue bulk copper complex single crystal; (the molar ratio of pyrazole-3-carboxylic acid ligand to copper acetate and lanthanum chloride is 6:1:1, the N,N-dimethylformamide solvent and distilled water The volume ratio is 1:2.5); yield: 69.4%.
Embodiment 3
[0025] Embodiment 3: Pyrazole carboxylic acid copper complex catalyzes the addition reaction of phenylacetylene to N-benzyl isatin:
[0026]
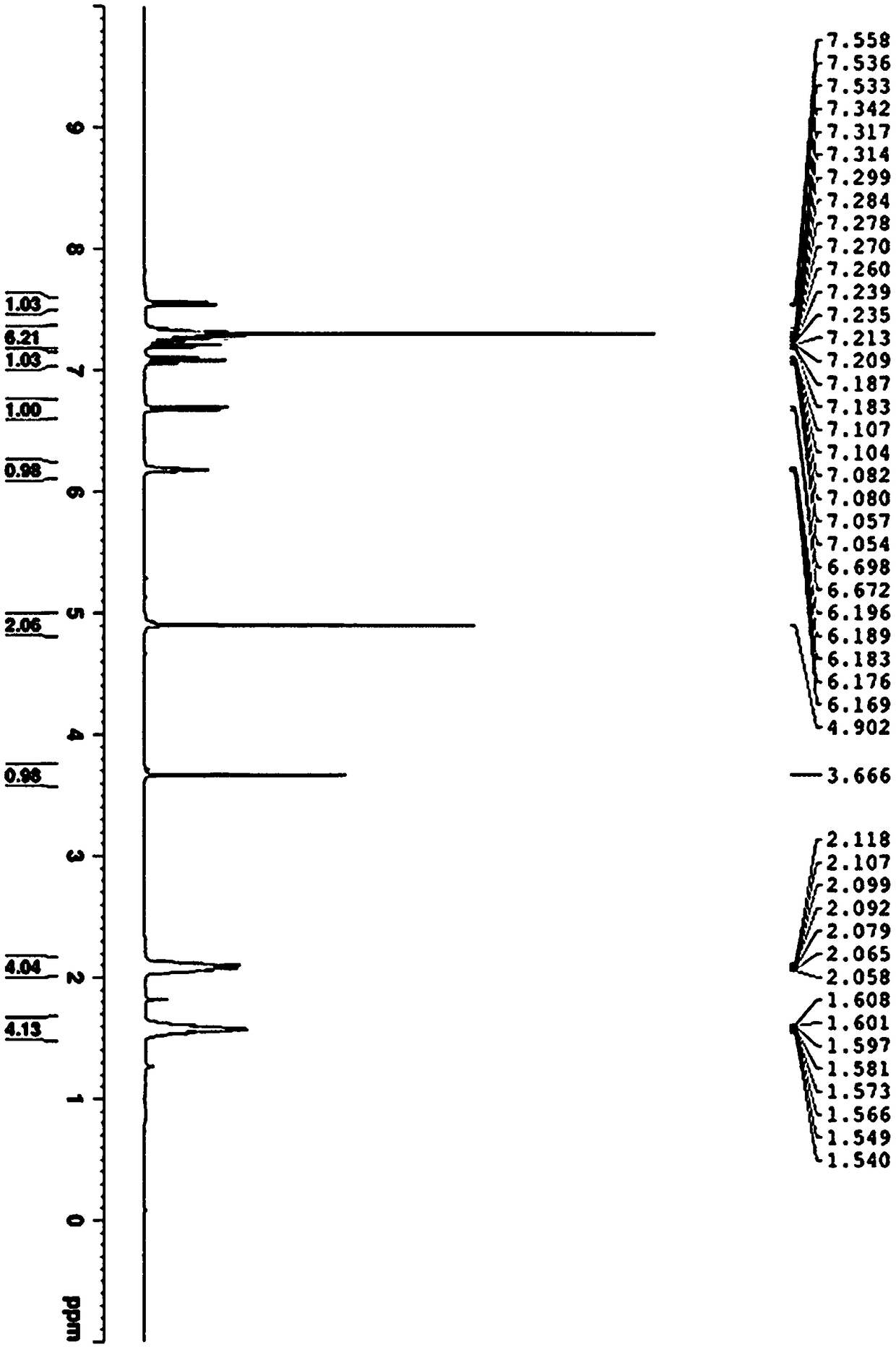
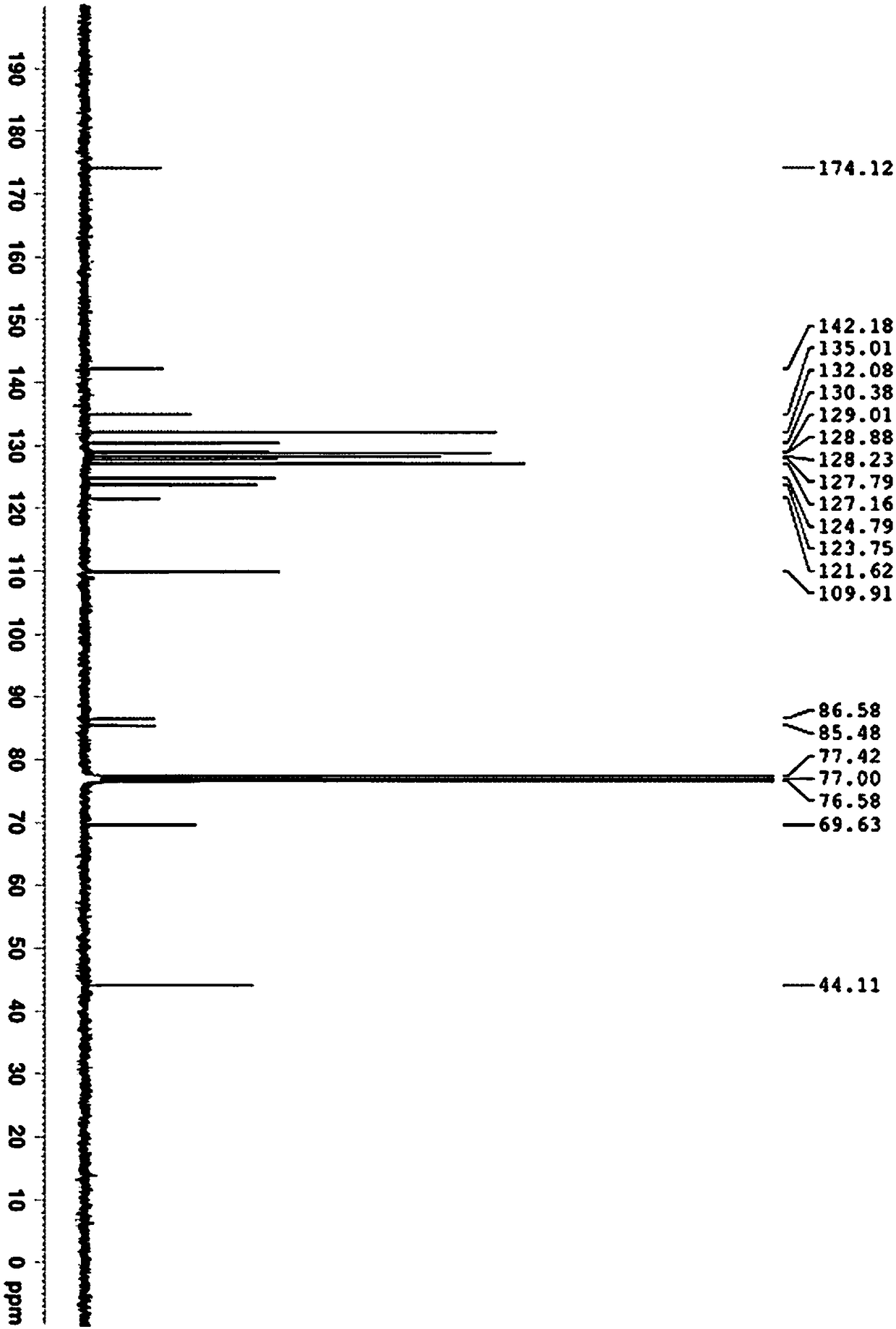
[0027] Dissolve 10mmol of phenylacetylene in 20mL of N,N-dimethylformamide solvent, add 0.2mmol of copper complex (recorded as copper) and then add 2.0mmol of triethylamine and 10mmol of N-benzyl isatin compound and mix well. The mixed reaction solution was placed at room temperature and stirred for 24 hours. After the reaction was completed, the solvent was removed under reduced pressure. After column chromatography, 3.18 g of the addition product M was obtained, with a yield of 94%. The proton nuclear magnetic spectrum data of compound M are as follows: (CDCl 3 ,300M Hz)δ=7.59-7.55(m,1H),7.43-7.01(m,12H),6.69-6.65(m,1H),4.84(s,2H),3.78(s,1H)ppm; compound M The carbon nuclear magnetic resonance spectrum data are as follows: (CDCl 3 ,75M Hz) δ=174.1, 142.2, 135.0, 132.1, 130.4, 129.0, 128.9, 128.2, 127.8, 127.2, 123.8, 121.6, 109.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com