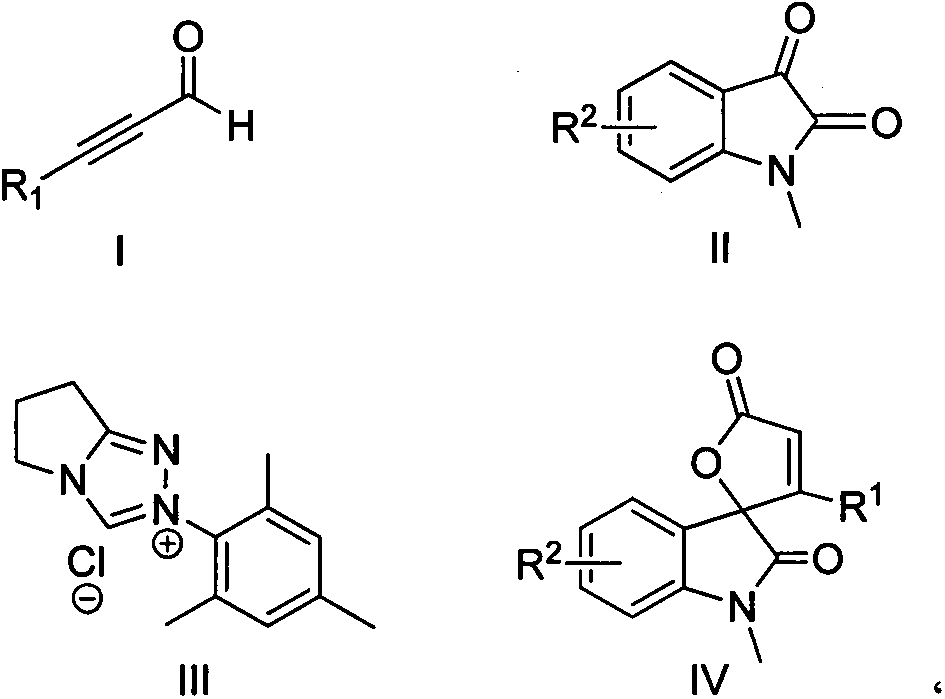
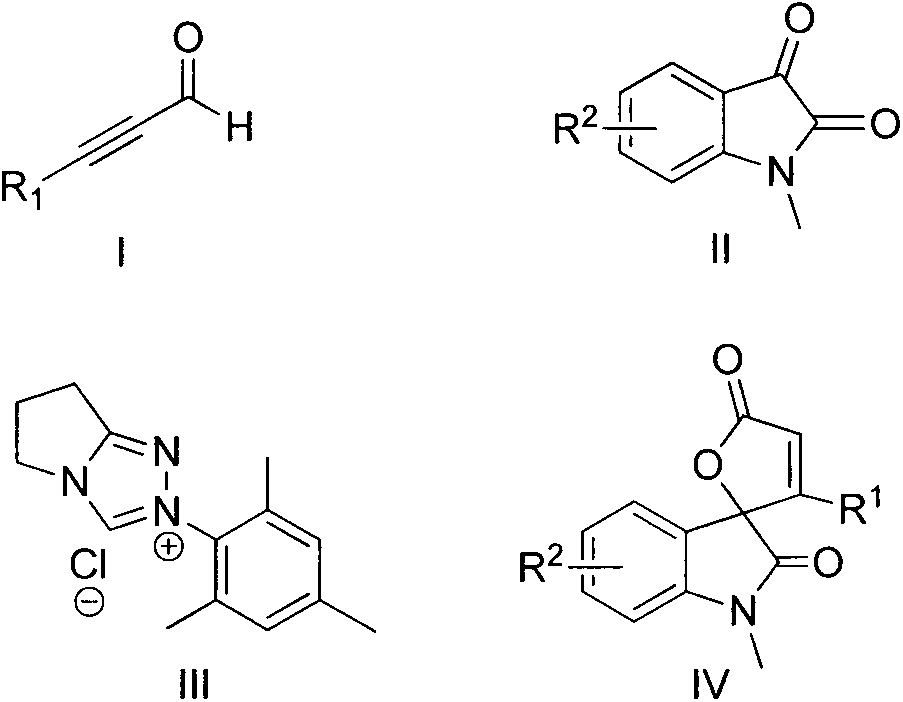
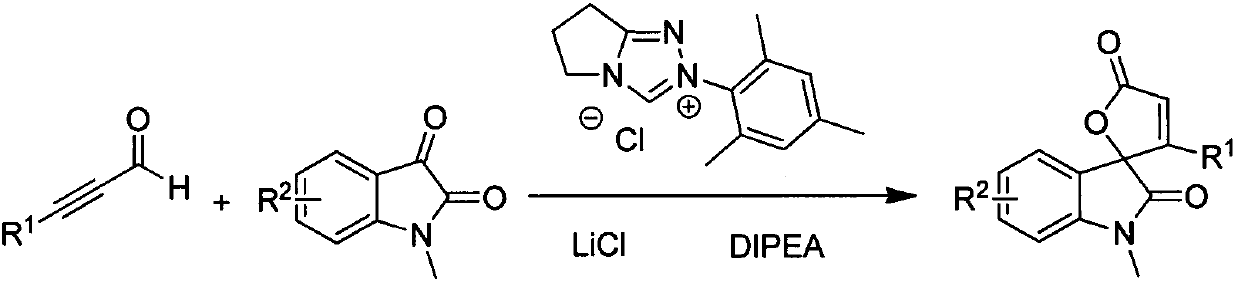
Synthesis method for spirally-epoxidized indole butenolide compound
A technology for indole croton lactone and compound is applied in the field of synthesizing spiro-epoxidized indole croton acid lactone compounds, and can solve the problems of less synthesis and research and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Embodiment 1: the reaction of 3-phenylpropional and 1-methyl isatin
[0013] 117mg (0.9mmol) of 3-phenylpropynaldehyde, 48.3mg (0.3mmol) of 1-methylisatin, 16mg (0.06mmol) of triazole salt as shown in formula III, and 38.1mg of diisopropylethylamine (0.3mmol), lithium chloride 14mg (0.33mmol) and dioxane 4mL were placed in a 25mL two-necked bottle, and reacted for 24h under nitrogen protection and 40°C. The reaction solution was cooled and concentrated. A mixed solvent with a ratio of 25:1 was used as the eluent for column chromatography to elute, and the eluate part of all detected products was collected, and 65 mg of the product was obtained after rotary evaporation to remove the solvent, with a yield of 75%.
[0014] White solid, MP: 194-195℃. 1 H NMR (300M, CDCl 3 ): δ7.46(m, 1H), 7.36(m, 1H), 7.24-7.29(m, 2H), 7.08-7.20(m, 4H), 6.99(d, J=7.8Hz, 1H), 6.66( s, 1H), 3.31(s, 3H). 13 C NMR (75M, CDCl 3 ): δ171.2, 169.9, 162.9, 144.4, 131.9, 131.5, 129.2, 128.9, 126...
Embodiment 2
[0015] Embodiment 2: the reaction of 3-(4-methylphenyl) propynaldehyde and 1-methyl isatin
[0016] 130mg (0.9mmol) of 3-(4-methylphenyl)propiolinal, 48.3mg (0.3mmol) of 1-methyl isatin, 16mg (0.06mmol) of triazole salt shown in formula III, diisopropyl 38.1mg (0.3mmol) of ethyl ethylamine, 14mg (0.33mmol) of lithium chloride and 4mL of dioxane were placed in a 25mL two-necked flask, and reacted for 24h under nitrogen protection and 65°C. A mixed solvent of petroleum ether: acetone with a volume ratio of 25:1 was used as an eluent for column chromatography, and the eluent part of all detected products was collected, and 65 mg of the product was obtained after rotary evaporation to remove the solvent, with a yield of 71%.
[0017] White solid, MP: 181-182℃. 1 H NMR (300M, CDCl3): δ7.44(t, J=7.8Hz, 1H), 7.15(d, J=7.5Hz, 1H), 6.96-7.09(m, 6H), 6.59(s, 1H), 3.27(s, 3H), 2.27(s, 3H). 13 C NMR (75M, CDCl 3 ): δ171.4, 170.0, 162.8, 144.3, 142.1, 131.8, 129.9, 126.9, 126.0, 124.9,...
Embodiment 3
[0018] Embodiment 3: the reaction of 3-(4-methoxyphenyl) propynaldehyde and 1-methyl isatin
[0019] 144mg (0.9mmol) of 3-(4-methoxyphenyl)propiolinal, 48.3mg (0.3mmol) of 1-methylisatin, 16mg (0.06mmol) of triazole salt shown in formula III, diiso 38.1mg (0.3mmol) of propylethylamine, 14mg (0.33mmol) of lithium chloride and 4mL of dioxane were placed in a 25mL two-neck flask, and reacted for 72h under nitrogen protection and 10°C, and the reaction solution was cooled and concentrated. After elution by column chromatography with a mixed solvent of petroleum ether: acetone volume ratio of 25:1 as the eluent, the eluent part of all the products detected was collected, and the product was obtained by rotary evaporation to remove the solvent 41mg, the yield was 42% .
[0020] White solid, MP: 200-201℃. 1 H NMR (300M, CDCl3): δ7.45(m, 1H), 6.98-7.17(m, 5H), 6.76(d, J=8.4Hz, 2H), 6.53(s, 1H), 3.75(s, 3H ), 3.30(s, 3H). 13 C NMR (75M, CDCl 3 ): δ171.5, 170.1, 162.3, 162.1, 144.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com