Synthetic method of spiro-oxoindole ethylene oxide derivative
A technology of indolinone oxirane and a synthesis method, applied in the direction of organic chemistry and the like, can solve problems such as poor adaptability of substrates, and achieve the effects of mild reaction conditions, simple operation and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055]
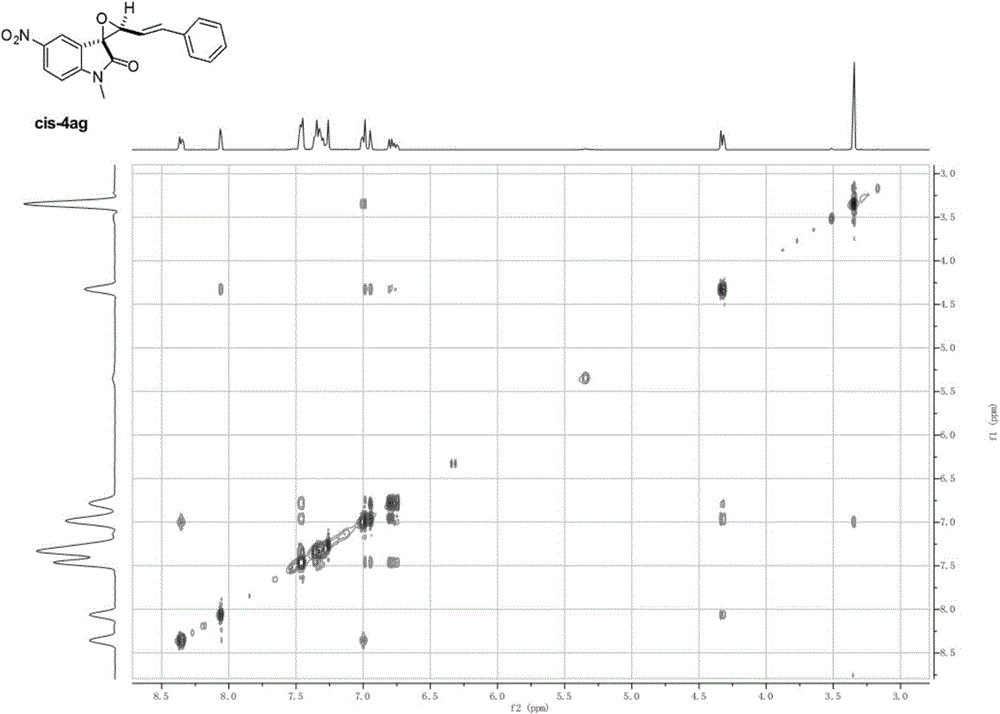
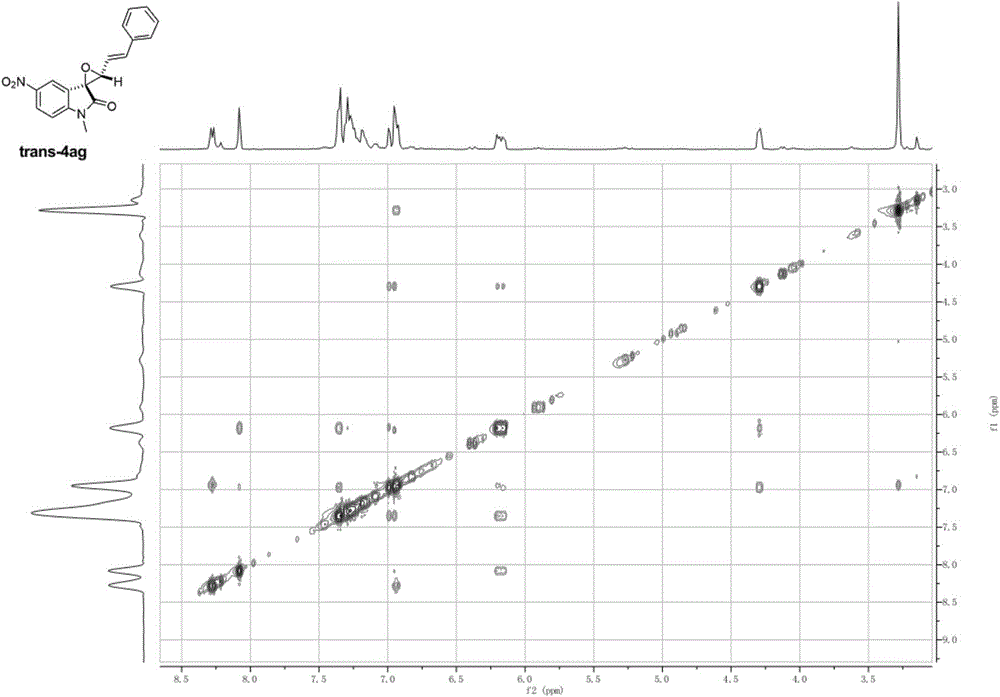
[0056] Add N-methylisatin (0.255mmol), p-tolylcinnamyl sulfide (0.306mmol), CsF (0.383mmol) and 8mL of anhydrous acetonitrile solvent into the reaction flask, stir at 40°C for 5min, and then add Add o-(trimethylsilyl)phenyl trifluoromethanesulfonate (0.765mmol) into the reaction flask, keep stirring at 40°C overnight, and remove the solvent by rotary evaporation at 40-45°C after the reaction to obtain the crude product, which is The pure product was obtained by column chromatography with a solvent with a volume ratio of ethyl acetate:petroleum ether=1:50-1:5, and the structure of the product was shown as cis-4aa and trans-4aa, and the nuclear magnetic resonance 1 H NMR, 13 The C NMR spectra are as follows Figure 4 , Figure 5 As shown, the total yield is 87%, cis / trans=44:56. The product cis-4aa: 1 H NMR (400MHz, CDCl 3 )δ7.49(dd, J=8.3, 2.0Hz, 1H), 7.47-7.42(m, 2H), 7.36-7.29(m, 3H), 7.27(d, J=2.0Hz, 2H), 6.92(d , J=16.2Hz, 1H), 6.80(dd, J=19.2, 8.4Hz, 2H), 4...
Embodiment 2
[0058]
[0059] Add 5-methyl-N-methyl isatin (0.255mmol), p-tolyl cinnamyl sulfide (0.306mmol), CsF (0.383mmol) and 8mL of anhydrous acetonitrile solvent into the reaction flask, at 40°C Stir for 5 minutes, then add o-(trimethylsilyl)phenyl trifluoromethanesulfonate (0.765mmol) into the reaction flask, keep stirring at 40°C overnight, and remove the solvent by rotary evaporation at 40-45°C after the reaction, to obtain crude Product,, the crude product is carried out column chromatography with the solvent that volume ratio is ethyl acetate:petroleum ether=1:50-1:5 and obtains pure product, and product structure is as shown in cis-4ab and trans-4ab, nuclear magnetic resonance 1 HNMR, 13 The C NMR spectra are as follows Figure 6 , Figure 7 As shown, the total yield is 50%, cis / trans=54:46. The product cis-4ab: Yellowish solid. 1 H NMR (400MHz, CDCl 3 ): δppm7.46(d, J=7.2Hz, 2H), 7.34-7.26(m, 3H), 7.17(d, J=8.0Hz, 1H), 6.98(s, 1H), 6.92-6.82(m, 2H), 6.79(d, J=8.0Hz, 1H)...
Embodiment 3
[0061]
[0062] Add 5-methoxy-N-methyl isatin (0.255mmol), p-tolyl cinnamyl sulfide (0.306mmol), CsF (0.383mmol) and 8mL of anhydrous acetonitrile solvent into the reaction flask, at 40 °C Stirring at low temperature for 5min, then adding o-(trimethylsilyl)phenyl trifluoromethanesulfonate (0.765mmol) into the reaction flask, keeping stirring at 40°C overnight, after the reaction was completed, the solvent was removed by rotary evaporation at 40-45°C to obtain The crude product, the crude product is subjected to column chromatography with a volume ratio of ethyl acetate:petroleum ether=1:50-1:5 to obtain pure product, the product structure is shown in cis-4ac and trans-4ac, NMR 1 H NMR, 13 The C NMR spectra are as follows Figure 8 , Figure 9As shown, the total yield is 32%, cis / trans=59:41. Product cis-4ac: 1H NMR (400MHz, CDCl3) δ7.46 (d, J=7.2Hz, 2H), 7.32 (dd, J=14.9 , 7.4Hz, 4H), 6.89(d, J=2.7Hz, 2H), 6.87-6.75(m, 2H), 4.17(d, J=7.6Hz, 1H), 3.81(s, 3H), 3.25(d , J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com