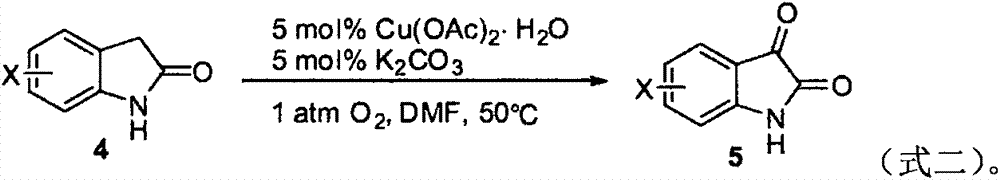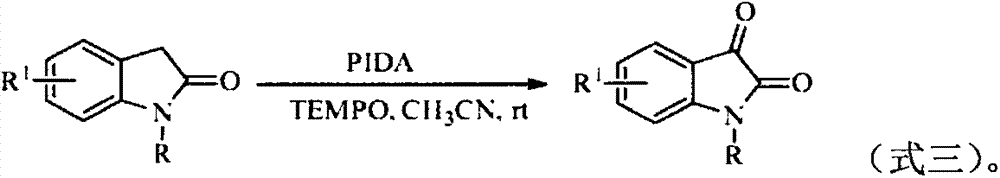Preparation method of isatin derivatives
A technology of derivatives and isatin, which is applied in the field of preparation of isatin derivatives, achieves the effect of low cost and simple and easy process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-12
[0025] Embodiment 1-12 Reaction condition optimization
[0026] Isatin (I-1) was prepared by reacting 2-indolinone (1a) with tert-butyl nitrite (2a) as the raw material. The influence of different reaction conditions on the reaction was explored, and a representative implementation was selected. Example 1-12, the results are shown in Table 1:
[0027]
[0028] Table I:
[0029] Example Auxiliary (equivalent) solvent Isolated yield 1 TBHP(2) THF 0 2 DTBP(2) THF 0 3 t-BuONO(2) THF 77 4 K 2 S 2 o 8 (2)
THF 5 5 t-BuONO(1.2) THF 61 6 t-BuONO(3) THF 76 7 a
t-BuONO(2) THF 28 8 b
t-BuONO(2) THF 0 9 t-BuONO(2) 1,4-dioxane 12 10 t-BuONO(2) EtOAc 75 11 t-BuONO(2) toluene 5 12 c
t-BuONO(2) THF 70
[0030] Taking Example 3 as an example, its specific operation is as follows: add 2-indolinone (1a, 0.3mmol), tert-butyl nitrite (t-BuONO, 2a, 0.6mm...
Embodiment 7
[0032] In embodiment 7, "a" represents that reaction is carried out under air atmosphere (1atm) condition;
Embodiment 8
[0033] "b" in Example 8 represents that the reaction is carried out under nitrogen atmosphere conditions;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com