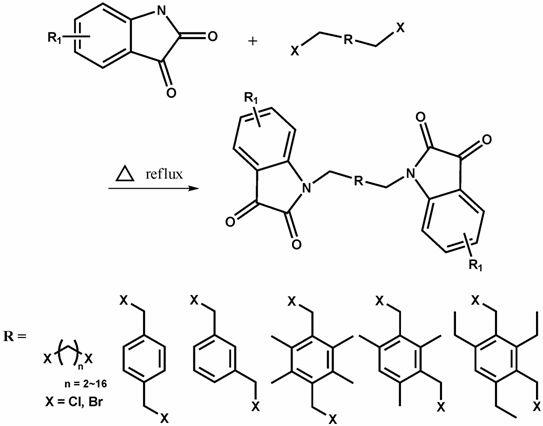
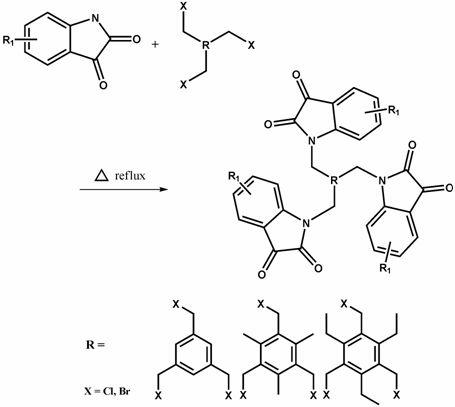
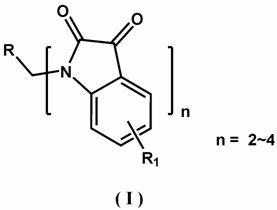
Multi-nitrogen substituted isatin derivative and synthetic method of multi-nitrogen substituted isatin derivative
A synthesis method and derivative technology are applied in the field of multi-nitrogen-substituted isatin derivatives and their synthesis, which can solve problems such as biological activity amplification, and achieve the effects of fast reaction speed, mild conditions and high purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Synthesis of 1a~1d (taking 1a2,4,6-trimethyl-1,3-dimethylene phenylenedisatin as an example)
[0028]
[0029] Weigh isatin 1.290 g (8.76 mmol), 1.046 g (3.00 mmol) 2,4-dibromomethyl-1,3,5 triethylbenzene, K 2 CO 3 1.008 g (7.30 mmol). Dissolve 1.290 g (8.76 mmol) of isatin in 40 ml of acetonitrile and stir at room temperature until the isatin is completely dissolved and the color of the solution is orange. Add the weighed K to it 2 CO 3 At this time, the color of the solution deepened and was dark red. After stirring for half an hour, 60 mL of acetonitrile solution dissolved in 2,4-dibromomethyl-1,3,5-triethylbenzene was added. Slowly raise the temperature to 60°C. The reaction was over in about 3 hours. The recrystallized product was an orange-red solid with a yield of 89.0%. ESI-MS m / z 503.5 ([m+NH 4 ] + , 100.0%). 1 H NMR (300 MHz, DMSO) δ 7.54 (d, J = 7.4, 1.1 Hz, 2H), 7.40 (t, J = 7.8, 1.4 Hz, 2H), 7.05 (d, J = 13.6, 6.0 Hz, 4H),...
Embodiment 2
[0030] Example 2: Synthesis of 2a~2d, 3a~3d (taking 2a2,4,6-trimethyl-1,3,5-trimethylenebenzenethree isatin as an example)
[0031]
[0032]
[0033]Weigh 1.80 g of isatin, 1.40 g of 1,3,5-tribromomethyl-2,4,6-trimethylbenzene, and 1.70 g of potassium carbonate were dissolved in 100 mL of acetonitrile. After stirring at 60° C. for 4 h, the reaction was stopped. A red solid was obtained by rotary evaporation under reduced pressure, which was dissolved by adding dichloromethane, and a large amount of potassium carbonate was filtered out. The filtrate was washed three times with water, anhydrous CaCl 2 dry. Spin-dry under reduced pressure to obtain a red solid. Column chromatography separation. The developing solvent is chloroform. Recrystallization, yield: 87.0%. 1 H NMR (300 MHz, DMSO) δ 7.50 (dd, J = 7.3, 1.2 Hz, 3H), 7.18 (td, J = 7.8, 1.3 Hz, 3H), 7.01 (dd, J = 17.4, 10.0 Hz, 3H), 6.51 (d, J = 7.9, Hz, 3H), 5.00 (s, 6H), 2.37 (s, 9H), ESI-HRMSm / z 620.2 ([...
Embodiment 3
[0034] Embodiment 3: Synthesis of 4a~4d (taking 4a1,2,4,5-tetratyl tetraisatin as an example)
[0035]
[0036] Weigh 1.80 g of isatin, 1.00 g of 1,2,4,5-tetrabromomethylbenzene, and 2.00 g of potassium carbonate and dissolve them in 80 mL of DMF. After stirring at 120°C for 24h, the reaction was stopped. Rotary evaporation under reduced pressure gave a red solid, which was dissolved by adding a large amount of dichloromethane, and filtered to remove potassium carbonate. The filtrate was washed three times with water, anhydrous CaCl 2 After drying overnight, it was spin-dried under reduced pressure to obtain a red powder. Column chromatography separation, developing solvent is chloroform. After recrystallization, yield: 60.0%. 1 H NMR (300 MHz, DMSO) δ 8.05 (dd, J = 7.3, 1.2 Hz, 4H), 7.65 (td, J = 7.8, 1.3 Hz, 4H), 7.47 (dd, J = 17.4, 10.0 Hz, 4H), 7.23 (d, J = 7.9 Hz, 4H), 6.75 (s, 2H), 4.63 (s, 8H), ESI-HRMSm / z 737.32 ([M+Na] + , 100%). m.p>300°C.
[0037] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com