Gronwell naphthaquinone derivative and its application in preparing anticancer medicine
A technology of naphthoquinones and derivatives is applied to naphthoquinone derivatives of shikonen and its application field in the preparation of anticancer drugs, and can solve the problem of insufficient antitumor activity and high
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The present invention will be further illustrated by the following examples. Example 1: Extraction, separation and purification of shikonin compounds
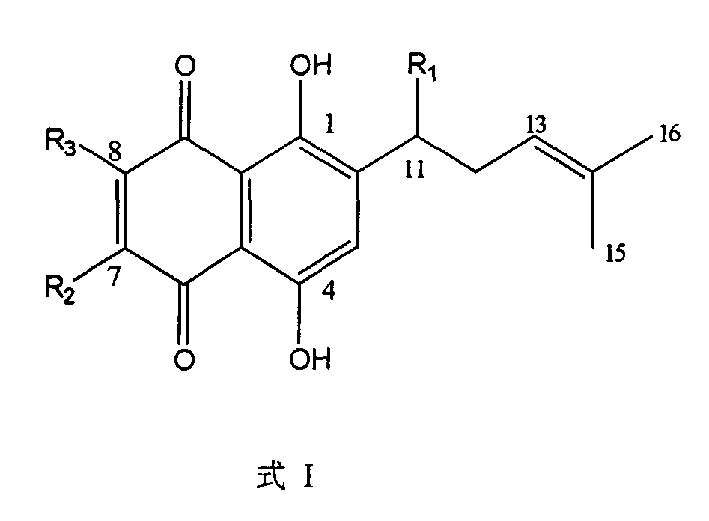
[0023] Shikonin compounds [R 1 =OH, (CH 3 ) 2 C=CHCOO, CH 3 COO or CH 3 COOC(CH 3 ) 2 CH 2 COO, R 2 =R 3 =H] The configuration is R-type, and the content is higher in Onosma confertum W.W. Smith.
[0024]Weigh 5 kg of Sichuan Lithospermum erythrorhizon, grind it through a 20-mesh sieve, and perform percolation extraction at room temperature with 95% ethanol until it is nearly colorless. The extracts were combined, concentrated under reduced pressure, and 2% NaOH equivalent to 1 / 3 to 1 / 2 of the volume of the concentrated solution was added to the concentrated solution, and the solution changed from purple-red to blue. After filtering to remove the insoluble matter, it was acidified by adding concentrated HCl. At this time, the solution changed from blue to purple-red, and a large amount of precipitate was formed at the same t...
Embodiment 2
[0024]Weigh 5 kg of Sichuan Lithospermum erythrorhizon, grind it through a 20-mesh sieve, and perform percolation extraction at room temperature with 95% ethanol until it is nearly colorless. The extracts were combined, concentrated under reduced pressure, and 2% NaOH equivalent to 1 / 3 to 1 / 2 of the volume of the concentrated solution was added to the concentrated solution, and the solution changed from purple-red to blue. After filtering to remove the insoluble matter, it was acidified by adding concentrated HCl. At this time, the solution changed from blue to purple-red, and a large amount of precipitate was formed at the same time. Let it stand overnight. The precipitate was filtered out and washed with distilled water to near neutral. The precipitate was dried to obtain about 60 g of a powdery solid crude product. Dissolve the crude product with dichloromethane, add appropriate amount of silica gel and stir evenly, and then evaporate to dryness before loading on the column. Th...
Embodiment 3
[0027] A methanol solution (8 mL) of 0.2 mmol β, β-dimethylacryloylakineine and 1 mmol aniline was stirred for reaction at room temperature for 6 days, and the reaction was followed by TLC. After the completion of the reaction, the solvent was removed under reduced pressure, and the crude product was separated by flash column chromatography (using petroleum ether-ethyl acetate as the eluent) to obtain a product in which the hydrogen at C-7 or C-8 was replaced by an arylamino group ( 9, 10), the total yield is about 80%. Using the same method, using β, β-dimethylacryloyl akanin as a raw material, compounds 11 and 12 can be synthesized. Embodiment four: R 1 Is substituted aniline, R 2 Or R 3 Synthesis of Shikonoquinone Derivatives Substituted for Phenylamino
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com