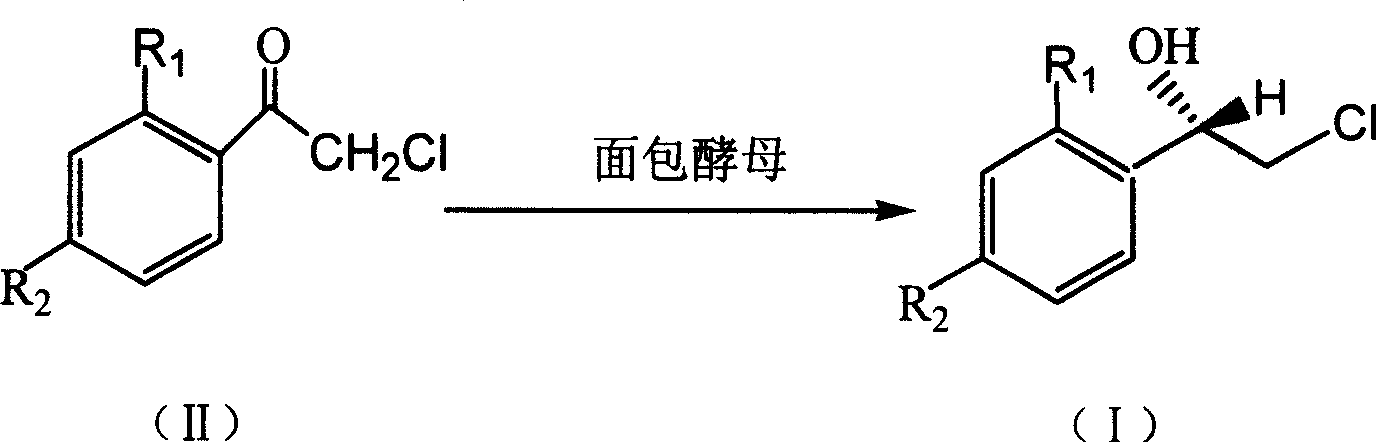
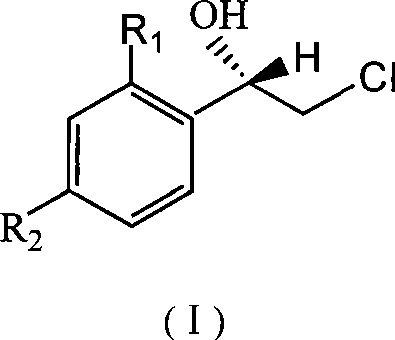
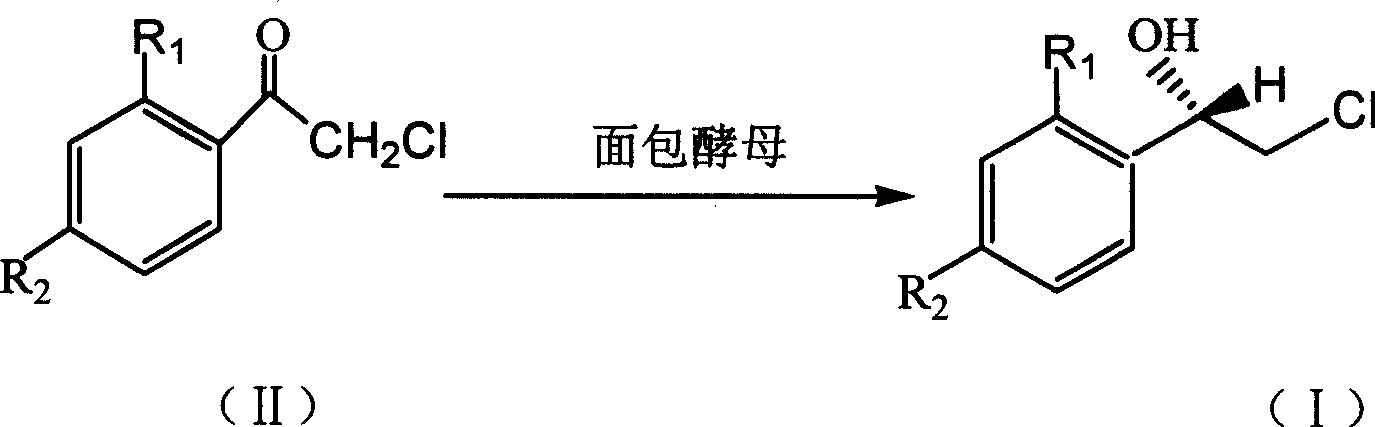
Method for asymmetric synthesis of (S)-2-chloro-1-phenylethanol derivative
A technology of phenylethanol and derivatives, applied in the field of biosynthesis, can solve the problems of high price and high cost of resolving agents, and achieve the effects of easy realization of reaction conditions, low cost and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 25 mL of pH 6.8 phosphate buffer, 3.0 g (120 g / L) of dry baker’s yeast and 1.25 g (50 g / L) of glucose into the Erlenmeyer flask, and vibrate at a certain temperature in a temperature-controlled shaker (140 r / L) min) 0.5h to activate the baker's yeast. Then add substrate 2,2', 4'-trichloroacetophenone 33.5mg (0.006mol / L) and auxiliary substrate methyl alcohol (5%, V 辅底物 / V 缓冲溶液 ), placed on a shaker (140r / min) at 30°C for 48 hours. After the reaction is over, add n-hexane to the reaction solution, place it in a shaker and extract it by shaking at 100r / min for 10 minutes, then transfer it to a separatory funnel, shake it again to make the extraction complete, and take the upper layer solution after standing for layers Carry out centrifugation, then take the supernatant after centrifugation and use anhydrous Na 2 SO 4 After drying, the substrate, product concentration and reaction stereoselectivity were analyzed by gas chromatography. The yield of the product (S)-...
Embodiment 2
[0024] Add 25 mL of pH 6.8 phosphate buffer, 2.5 g (100 g / L) of dry baker’s yeast and 1.25 g (50 g / L) of glucose into the Erlenmeyer flask, and vibrate at a certain temperature in a temperature-controlled shaker (140 r / L). min) 0.5h to activate the baker's yeast. Then add substrate 2,2', 4'-trichloroacetophenone 55.9mg (0.01mol / L) and auxiliary substrate ethanol (5%, V 辅底物 / V 缓冲溶液 ), placed on a shaker (140r / min) at 30°C for 24 hours. After the reaction is over, add n-hexane to the reaction solution, place it in a shaker and extract it by shaking at 100r / min for 10 minutes, then transfer it to a separatory funnel, shake it again to make the extraction complete, and take the upper layer solution after standing for layers Carry out centrifugation, then take the supernatant after centrifugation and use anhydrous Na 2 SO 4 After drying, the substrate, product concentration and reaction stereoselectivity were analyzed by gas chromatography. The yield of the product (S)-2-chlor...
Embodiment 3
[0026] Add 25 mL of pH 7.0 phosphate buffer, 2.25 g (90 g / L) of dry baker’s yeast and 1.25 g (50 g / L) of glucose into the Erlenmeyer flask, and vibrate at a certain temperature in a temperature-controlled shaker (140 r / L). min) 0.5h to activate the baker's yeast. Then add substrate 2,2', 4'-trichloroacetophenone 335.3mg (0.06mol / L) and auxiliary substrate methyl alcohol (5%, V 辅底物 / V 缓冲溶液 ), placed on a shaker (140r / min) at 30°C for 36 hours. After the reaction is over, add n-hexane to the reaction solution, place it in a shaker and extract it by shaking at 100r / min for 10 minutes, then transfer it to a separatory funnel, shake it again to make the extraction complete, and take the upper layer solution after standing for layers Carry out centrifugation, then take the supernatant after centrifugation and use anhydrous Na 2 SO 4 After drying, the substrate, product concentration and reaction stereoselectivity were analyzed by gas chromatography. The yield of the product (S)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com