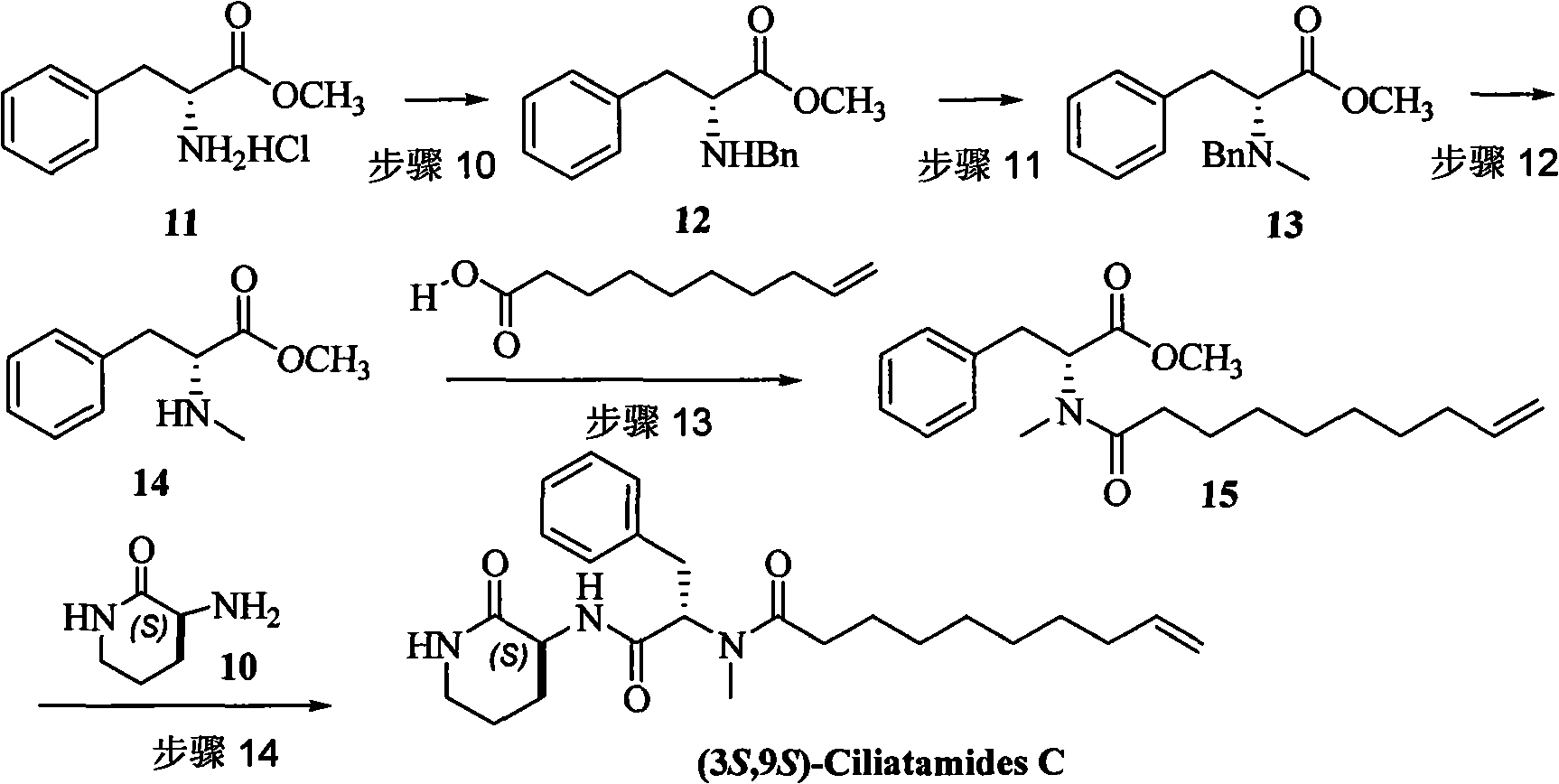
Method for preparing natural product (3S,9S)-Ciliatamides C
A technology for natural products and compounds, applied in organic chemistry and other directions, can solve problems such as high price, poor amino selectivity, and product racemization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
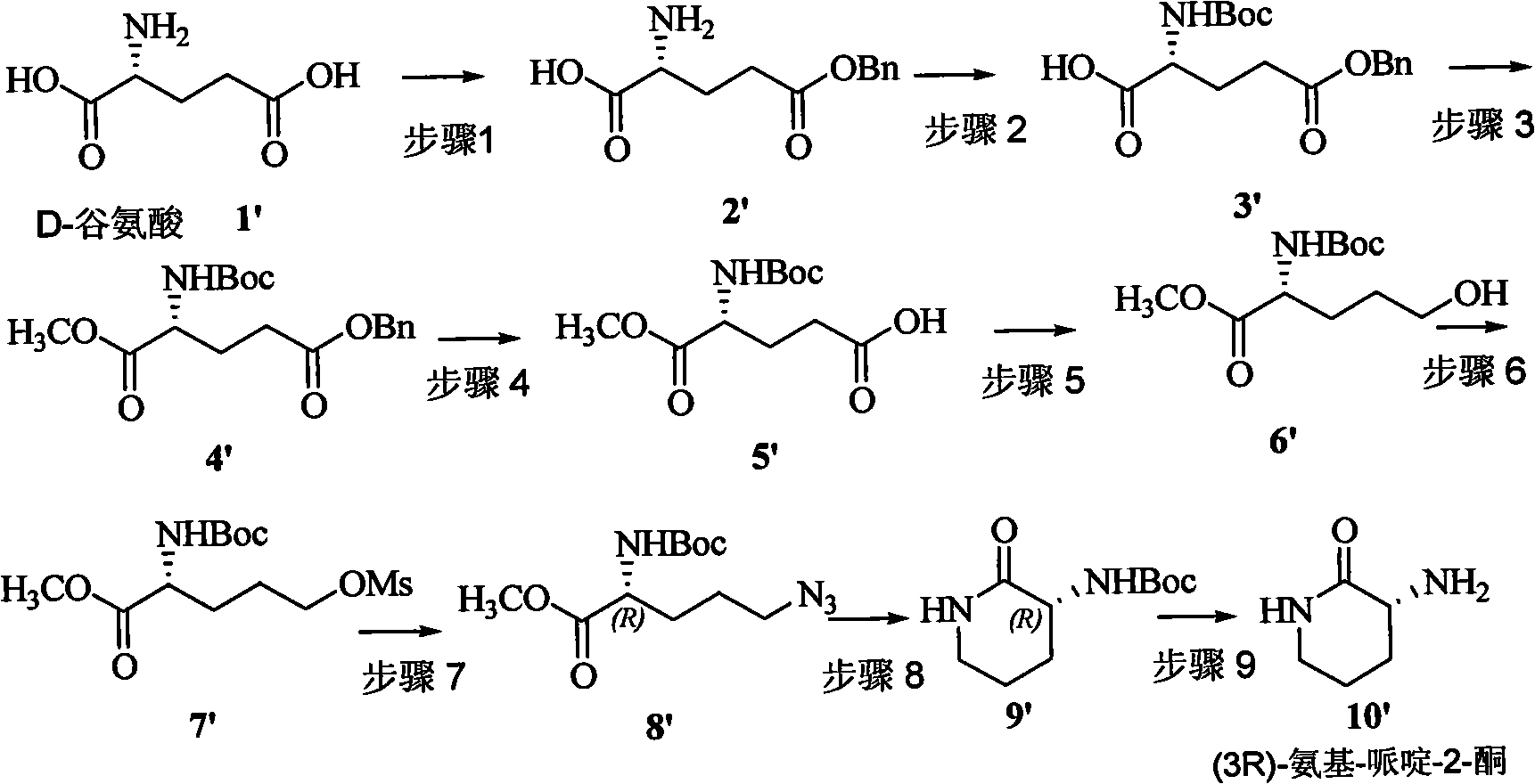
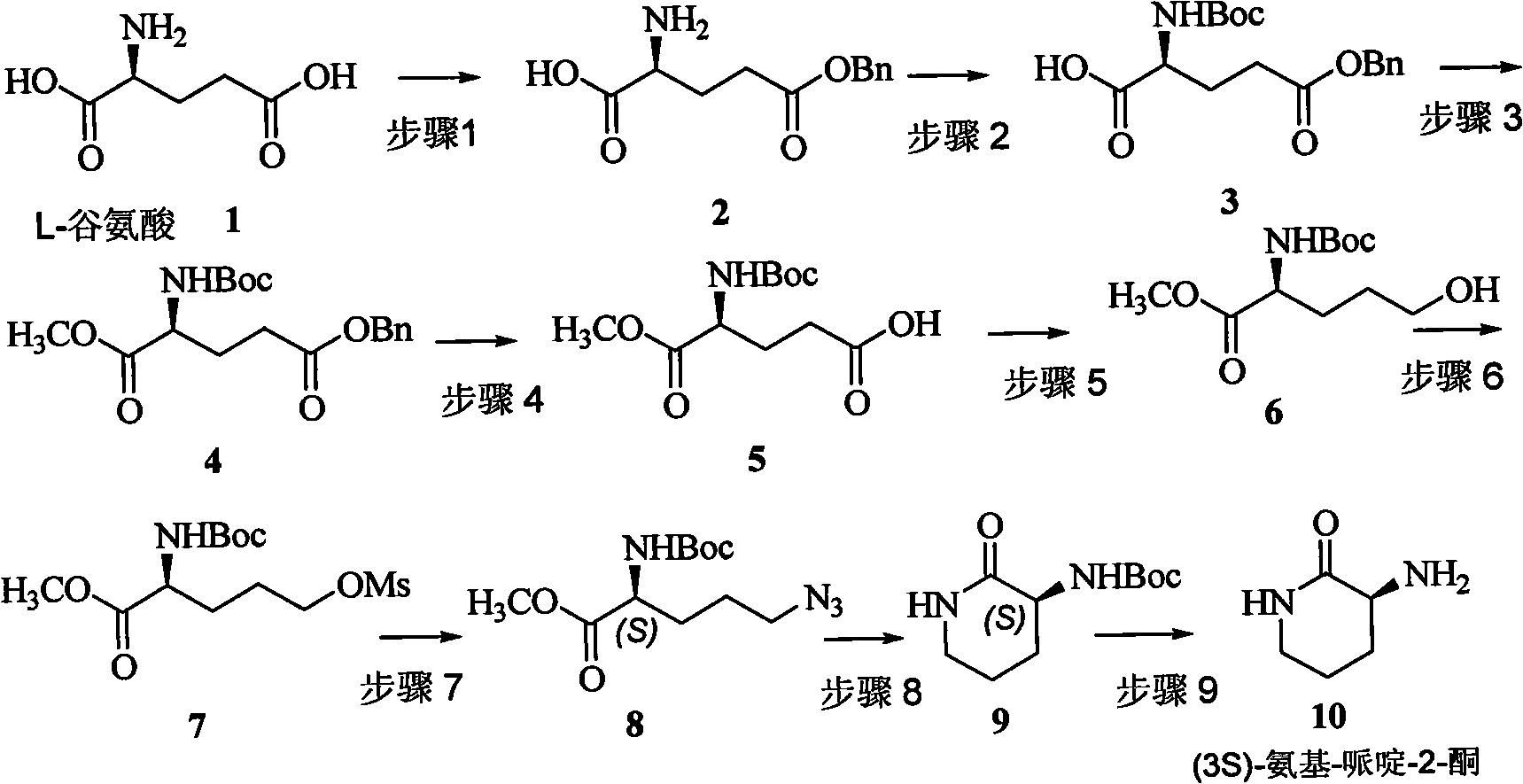
[0039] Step 1 Synthesis of (S)-2-amino-5-(benzyloxy)carboxy-pentanoic acid 2
[0040] The compound was purchased directly from the market or prepared according to the literature: that is, under nitrogen protection, benzyl alcohol (34.0 mmol) and 60% concentrated sulfuric acid (27.2 mmol) were added to L-glutamic acid (1, 27.2 mmol) at 70°C. Vacuum dehydration for 6 hours. The reaction system was quenched by adding saturated sodium bicarbonate (27.2 mmol) overnight, and filtered to obtain compound 2 (40-50.0%).
[0041] Step 2 Synthesis of (S)-5-(benzyloxy)carboxy-2-tert-butoxycarbonylamino--pentanoic acid 3
[0042] Compound 2 (50 mmol) was dissolved in a mixed solvent of 150 mL of dioxane and 150 mL of water, di-tert-butyl carbonate and triethylamine were added at 0 °C, reacted at 0 °C to rt. for 12 hours, concentrated and diluted with water, Extracted twice with ether, acidified the aqueous phase with 5 mol / L hydrochloric acid to pH=1 and extracted three times with ethyl a...
Embodiment 2
[0067] Step 1 Synthesis of (R)-2-amino-5-(benzyloxy)carboxy-pentanoic acid 2'
[0068] The compound was purchased directly from the market or prepared according to the literature: that is, under nitrogen protection, benzyl alcohol (34.0 mmol) and 60% concentrated sulfuric acid (27.2 mmol) were added to D-glutamic acid (1', 27.2 mmol) at 70°C. Dehydrated under vacuum for 6 hours. The reaction system was quenched by adding saturated sodium bicarbonate (27.2 mmol) overnight, and filtered to obtain compound 2' (40-50.0%).
[0069] Step 2 Synthesis of (R)-5-(benzyloxy)carboxy-2-tert-butoxycarbonylamino--pentanoic acid 3'
[0070] The synthesis of compound 3' was the same as in step 2 described in Example 1.
[0071] Step 3 Synthesis of (R)-5-(benzyloxy)-1-methyl-2-tert-butoxycarbonylamino-glutaric acid diester 4' Synthesis of Compound 4' and Step 3 described in Example 1 same.
[0072] Step 4 Synthesis of (R)-4-tert-butoxycarbonylamino-5-(methoxy)carboxy-pentanoic acid 5'
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com