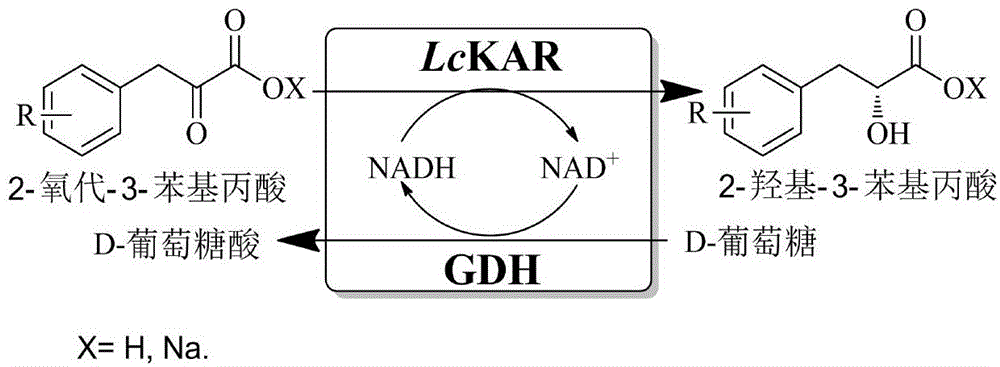
Phenylpyruvate reductase and application thereof to asymmetric synthesis of (R)-phenyllactic acid
A technology of phenylpyruvate and reductase, which is applied in the field of bioengineering, can solve the problems of low yield of phenyllactic acid, difficulty in extraction, and inability to achieve industrial production, and achieve the effects of environmental friendliness, good optical purity, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Screening of Lactobacillus CGMCC No.9967
[0033] (1) Using phenylpyruvate as a substrate, a strain of Lactobacillus JNU-KR1002, which can efficiently catalyze the reduction of phenylpyruvate to (R)-phenyllactate, was isolated from Mashan, Wuxi, with a preservation number of CGMCC 9967.
[0034] (2) Lactobacillus CGMCC No.9967 catalyzes the asymmetric reduction of phenylpyruvate
[0035] Lactobacillus CGMCC No.9967 in MRS medium (beef extract 10g / L, peptone 10g / L, yeast extract powder 5g / L, glucose 20g / L, sodium acetate 5g / L, diammonium citrate 2g / L, magnesium sulfate 0.58g / L, manganese sulfate 0.28g / L, Tween 800.1g / L, dipotassium hydrogen phosphate 2g / L, pH 6.2-6.4) at 37°C for 24 hours. Gained culture fluid obtains thalline precipitation through centrifugation, with the wet cell of 10g / L described lactobacillus as biocatalyst, when substrate concentration is 10mM, can make the conversion rate of phenylpyruvate reach 90% in 12 hours, product The yield was 1...
Embodiment 2
[0038] Cloning of Example 2 Ketoacid Reductase Gene
[0039] The phenylpyruvate reductase gene in the above Lactobacillus CGMCC No.9967 was cloned by the shotgun method.
[0040] (1) First, the above-mentioned Lactobacillus CGMCC No.9967 was mixed with beef extract 10g / L, peptone 10g / L, yeast extract powder 5g / L, glucose 20g / L, sodium acetate 5g / L, diammonium citrate 2g / L , magnesium sulfate 0.58g / L, manganese sulfate 0.28g / L, Tween 800.1g / L, dipotassium hydrogen phosphate 2g / L, pH 6.2-6.4 culture medium at 37°C for 24 hours. The resulting culture solution was centrifuged to obtain bacterial pellets, and the total DNA was extracted by conventional techniques in the art.
[0041] (2) The resulting total DNA can be digested with restriction endonucleases to form specific sticky ends, for example, GATC sticky ends can be formed by Sau3AI digestion. By controlling the enzyme dosage and reaction time, the total DNase is cut into 2-6kb fragments and recovered by enzyme digestion. ...
Embodiment 3
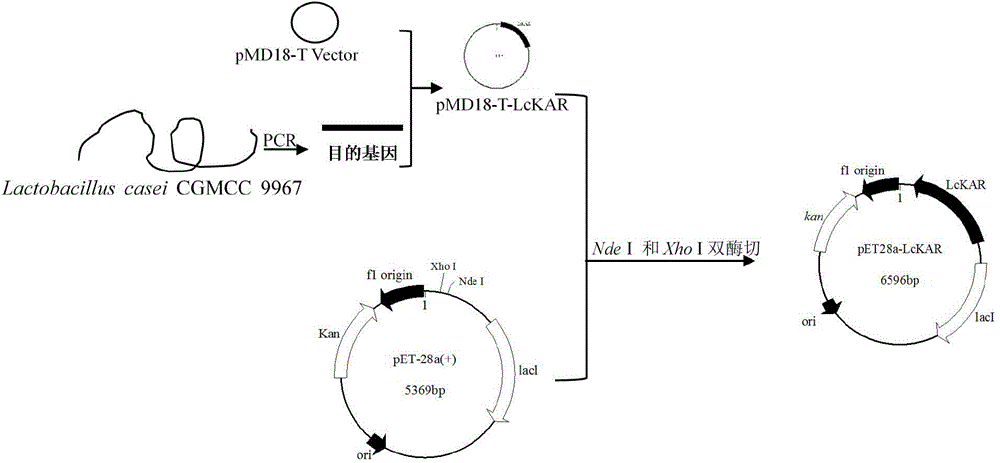
[0048] Example 3 Construction and recombinant expression of recombinant plasmid pET28a-LcKAR
[0049] The phenylpyruvate reductase gene LcKAR target band recovered in Example 2 was double-digested with restriction endonucleases Nde I and Xho I at 37°C for 9 hours, purified by agarose electrophoresis, and then purified using an agarose gel DNA recovery kit Recycle the target fragment. Under the action of T4 DNA ligase, the target fragment was ligated with the plasmid pET28a that was also double-digested with restriction endonucleases Nde I and Xho I, and then ligated overnight at 4°C to obtain the recombinant plasmid pET28a-LcKAR( image 3 ). Transform the recombinant plasmid pET28a-LcKAR into E.coli BL21(DE3) competent, spread the transformation solution on an LB plate containing 50 mg / L kanamycin, culture it upside down at 37°C overnight, and pick a single colony for PCR And enzyme activity verification, the positive recombinant bacteria E.coli BL21(DE3) / pET28a-LcKAR was ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com