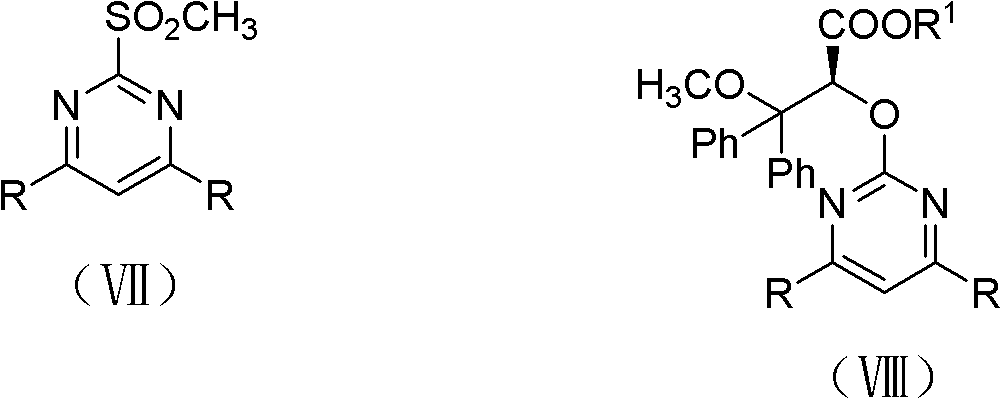A preparation method of optically pure (+)-ambisentan and optically pure (+)-dalusentan
A compound, the technology of diphenyl acrylate, is applied in the field of preparation of ambesentan and darusentan, which can solve the problems of increased cost, low utilization rate of raw materials, and restrictions on the application of industrial scale of products, so as to increase the income rate, the effect of reducing content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
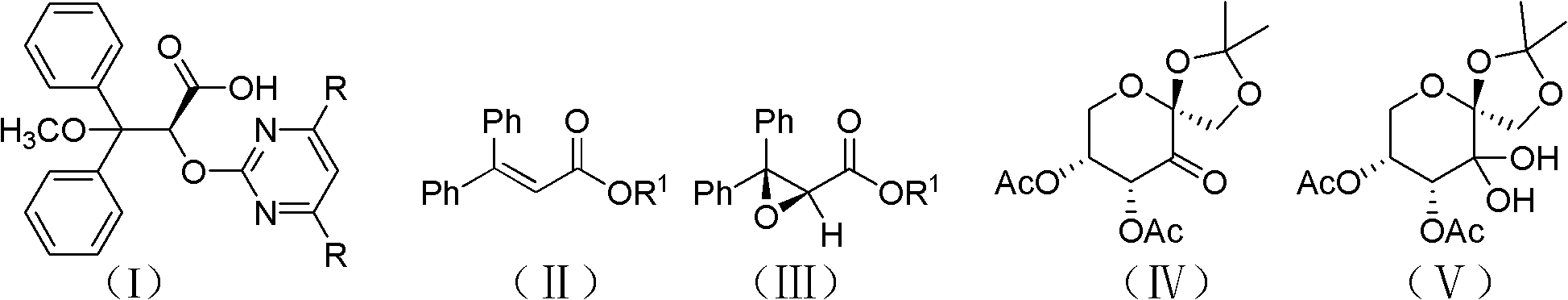
[0034] Example 1, (+)-Ambsentan ((2S)-2-[(4,6-dimethylpyrimidin-2-yl)-oxygen]-3-methoxy-3,3-diphenyl propionic acid) preparation
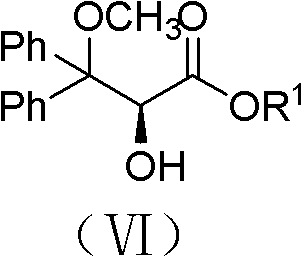
[0035] (1) Preparation of (2S)-3,3-diphenyl-2,3-epoxypropionic acid ethyl ester
[0036]
[0037] The reaction equation is shown in the above formula, wherein, Ph is phenyl; Ac is acetyl;
[0038] Add 3,3-ethyl diphenylacrylate (0.536mol, 135.0g) dissolved in 3.0L acetonitrile to a 50L reaction kettle with a mechanical stirrer, and dissolve in 1.5L acetonitrile to obtain a concentration of 0.12M Fructose-derived chiral ketone shown in formula (IV), and adding tetra-n-butyl ammonium bisulfate (36mmol, 12.2g), then adding 3.0L 1×10 -4 M's disodium ethylenediaminetetraacetic acid aqueous solution; put cooling liquid in the interlayer of the reaction kettle, adjust the temperature in the reaction kettle to -5°C-+5°C; 1.85kg of potassium monopersulfate compound salt and 0.78kg NaHCO 3 (9.29mol) of the mixture, it takes about 4.5 hours to add th...
Embodiment 2
[0052] Example 2, (+)-dalusentan ((2S)-2-[(4,6-dimethoxypyrimidin-2-yl)-oxygen]-3-methoxy-3,3-diphenyl Propionic acid) preparation
[0053] (1) Preparation of (2S)-3,3-diphenyl-2,3-epoxypropionic acid ethyl ester
[0054]
[0055] The reaction equation is shown in the above formula, wherein, Ph is phenyl; Ac is acetyl;
[0056] Add 3,3-diphenyl acrylate ethyl ester (0.267mol, 67.3g) dissolved in 1.5L acetonitrile in the 50L reaction kettle with mechanical stirrer, the concentration obtained in 0.75L acetonitrile is 0.12M Fructose-derived chiral ketone shown in formula (IV), and adding tetra-n-butyl ammonium bisulfate (18mmol, 6.1g), then adding 1.5L 1×10 -4 M's disodium ethylenediaminetetraacetic acid aqueous solution; put cooling liquid in the interlayer of the reaction kettle, adjust the temperature in the reaction kettle to -5°C-+5°C; 0.923kg (1.5mol) potassium hydrogen persulfate compound salt and 0.391 kg NaHCO 3 (4.65mol) of the mixture, it takes about 4.5 hours...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com