Sulfur recovery process for reducing SO2 emission
A sulfur dioxide and sulfur recovery technology, which is applied in the preparation/purification of sulfur, inorganic chemistry, sulfur compounds, etc., can solve problems such as failure to meet emission standards and increase emission concentration, and achieve significant environmental benefits, emission concentration reduction, and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
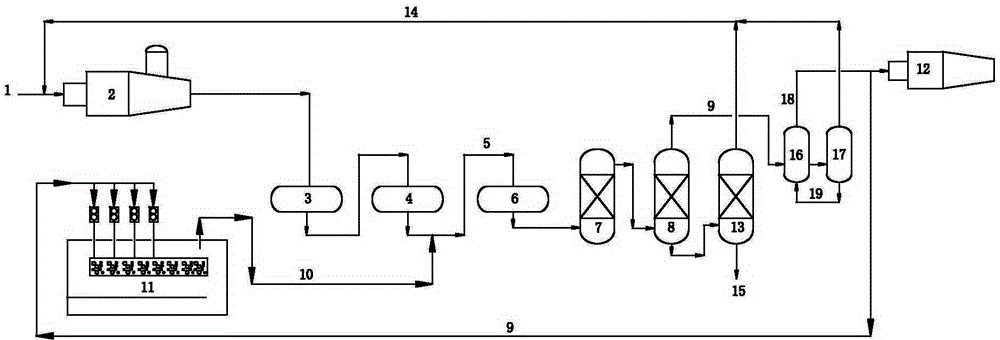
[0045] process such as figure 1 shown. The process includes thermal reaction section, catalytic reaction section and tail gas purification treatment section.
[0046] The thermal reaction section contains H 2 About 92 (v / v)% of S acid gas 1 is partially combusted into SO in reactor 2 2 , at a high temperature of 1250 °C for H 2 S and SO 2 A Claus reaction occurs to form elemental sulfur and process gas. The volume content of sulfur compounds in the process gas is: H 2 S: 5.8%, SO 2 : 2.6%, organic sulfur: 0.5%.
[0047] The process gas enters the primary converter 3 of the catalytic reaction section (reaction conditions: temperature 310°C, space velocity 800h -1 ) and secondary converter 4 (reaction conditions: temperature 245°C, space velocity 800h -1 ), after Claus catalytic conversion, the reacted Claus tail gas 5 enters the tail gas purification unit. The volume content of sulfur compounds in Claus tail gas is: H 2 S: 2.9%, SO 2 : 1.3%, organic sulfur: 0.1%.
...
Embodiment 2
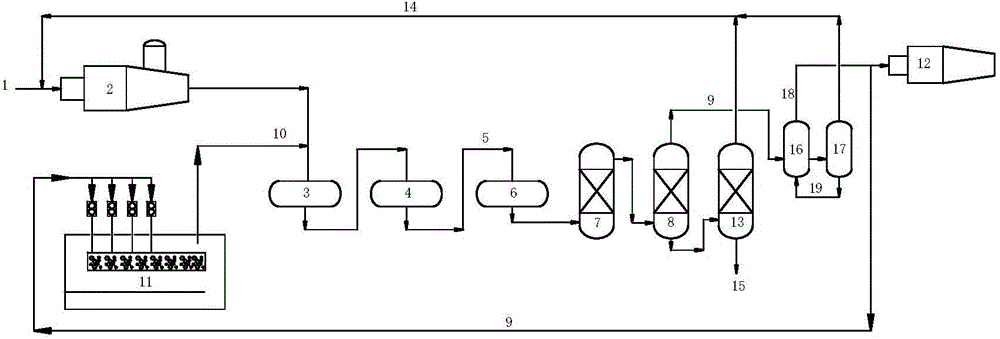
[0050] process such as figure 1 shown. The process includes thermal reaction section, catalytic reaction section and tail gas purification treatment section.
[0051] The thermal reaction section contains H 2 About 82 (v / v)% of S acid gas 1 is partially combusted into SO in reactor 2 2 , at a high temperature of 1250 °C for H 2 S and SO 2 A Claus reaction occurs to form elemental sulfur and process gas. The volume content of sulfur compounds in the process gas is: H2 S: 5.6%, SO 2 : 2.4%, organic sulfur: 0.4%.
[0052] The process gas enters the primary converter 3 in the catalytic reaction section (reaction conditions: temperature 300°C, space velocity 800h -1 ) and secondary converter 4 (reaction conditions: temperature 250°C, space velocity 800h -1 ), after Claus catalytic conversion, the reacted Claus tail gas 5 enters the tail gas purification unit. The volume content of sulfur compounds in Claus tail gas is: H 2 S: 2.7%, SO 2 : 1.3%, organic sulfur: 0.09%.
...
Embodiment 3
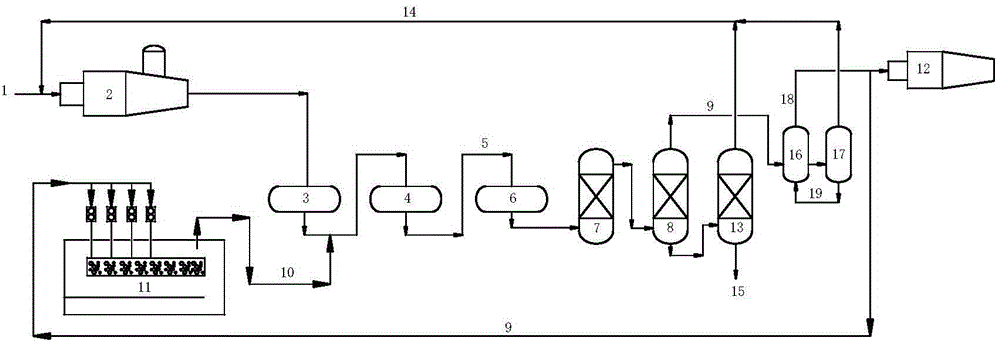
[0055] process such as figure 1 shown. The process includes thermal reaction section, catalytic reaction section and tail gas purification treatment section.
[0056] The thermal reaction section contains H 2 About 82 (v / v)% of S acid gas 1 is partially combusted into SO in reactor 2 2 , at a high temperature of 1250 °C for H 2 S and SO 2 A Claus reaction occurs to form elemental sulfur and process gas. The volume content of sulfur compounds in the process gas is: H 2 S: 5.6%, SO 2 : 2.4%, organic sulfur: 0.4%.
[0057] The process gas enters the primary converter 3 in the catalytic reaction section (reaction conditions: temperature 300°C, space velocity 800h -1 ) and secondary converter 4 (reaction conditions: temperature 250°C, space velocity 800h -1 ), after Claus catalytic conversion, the reacted Claus tail gas 5 enters the tail gas purification unit. The volume content of sulfur compounds in Claus tail gas is: H 2 S: 2.7%, SO 2 : 1.3%, organic sulfur: 0.09%. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com