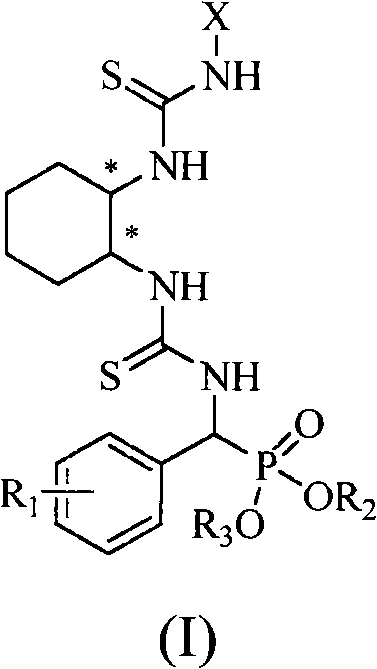
A chiral bithioureido derivate containing phosphonate as well as its preparing method and use
A dithiourea derivative, chiral technology, applied in the field of chiral dithiourea derivative and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
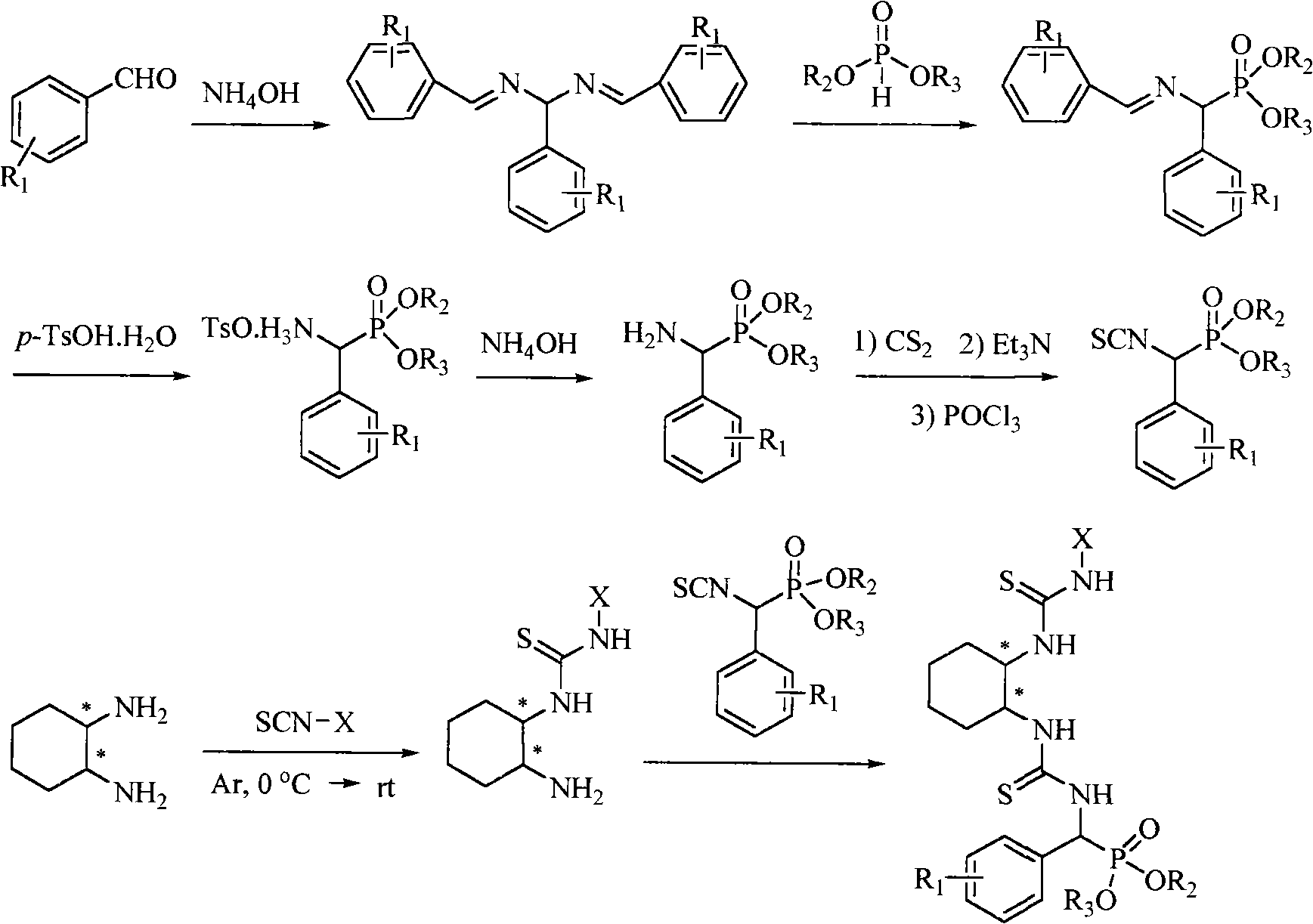
[0042] Embodiment 1, compound O, O'-diethylphenyl (3-((1R, 2R)-2-(3-phenylthioureido) cyclohexyl) thioureido) methylphosphonate (compound Synthesis numbered as a):
[0043] (1) O, the synthesis of O'-diethylamino (phenyl) methyl phosphonate:
[0044] Benzaldehyde (15mmol, 1.59g) and ammonia water (26%, 30mL) were added into a 50mL round bottom flask, and stirred at reflux for 3h. During this process, a white precipitate formed, which was filtered and dried. Subsequently, a 100mL three-neck round bottom flask was taken, and the dried solid was added to it, followed by diethyl phosphite (7.5mmol, 1.04g), the solid was dissolved, and refluxed at 70°C for 5h, during which an intermediate was produced. Afterwards, p-toluenesulfonic acid (5 mmol, 0.86 g) dissolved in 50 mL of tetrahydrofuran was added dropwise to the above reaction system, and stirred at 0° C. for 2 h. A large amount of solid formed which was filtered and washed with 20 mL of tetrahydrofuran. Take a 100mL round ...
Embodiment 2
[0051] Example two, compound O, O'-di-n-propylphenyl (3-((1R, 2R)-2-(3-phenylthioureido) cyclohexyl) thioureido) methylphosphonate ( Compound number is the synthesis of b):
[0052] (1) O, the synthesis of O'-di-n-propylamino (phenyl) methyl phosphonate:
[0053] Synthesize as in Example 1 (1) method and conditions. The difference is that di-n-propyl phosphite (7.5mmol, 1.25g) was added, the reaction time was 6h, and the yield was 45.9%.
[0054] (2) O, the synthesis of O' di-n-propyl isothiocyanate (phenyl) methyl phosphonate:
[0055] Synthesize as in Example 1 (2) method and conditions. The difference is that O, O'-di-n-propylamino(phenyl)methylphosphonate (6mmol, 1.63g) was added, and the yield was 61.5%.
[0056] (3) Synthesis of 1-((1R, 2R)-2-aminocyclohexyl)-3-phenylthiourea:
[0057] Synthesize as in Example 1 (3) method and conditions.
[0058] (4) Synthesis of O, O'-di-n-propylphenyl (3-((1R, 2R)-2-(3-phenylthioureido) cyclohexyl) thioureido) methylphosphonate:...
Embodiment 3
[0060] Example three, compound O, O'-diisopropylphenyl (3-((1R, 2R)-2-(3-phenylthioureido) cyclohexyl) thioureido) methylphosphonate ( Compound number is the synthesis of c):
[0061] (1) O, the synthesis of O'-diisopropylamino (phenyl) methyl phosphonate:
[0062] Synthesize as in Example 1 (1) method and conditions. The difference is that diisopropyl phosphite (7.5mmol, 1.25g) was added, the reaction time was 6h, and the yield was 50.3%.
[0063] (2) O, the synthesis of O'-diisopropyl isothiocyanate (phenyl) methyl phosphonate:
[0064] Synthesize as in Example 1 (2) method and conditions. The difference is that O,O'-diisopropylamino(phenyl)methylphosphonate (6mmol, 1.63g) was added, and the yield was 54.2%.
[0065] (3) Synthesis of 1-((1R, 2R)-2-aminocyclohexyl)-3-phenylthiourea:
[0066] Synthesize as in Example 1 (3) method and conditions.
[0067] (4) Synthesis of O, O'-diisopropylphenyl (3-((1R, 2R)-2-(3-phenylthioureido) cyclohexyl) thioureido) methylphosphonate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com