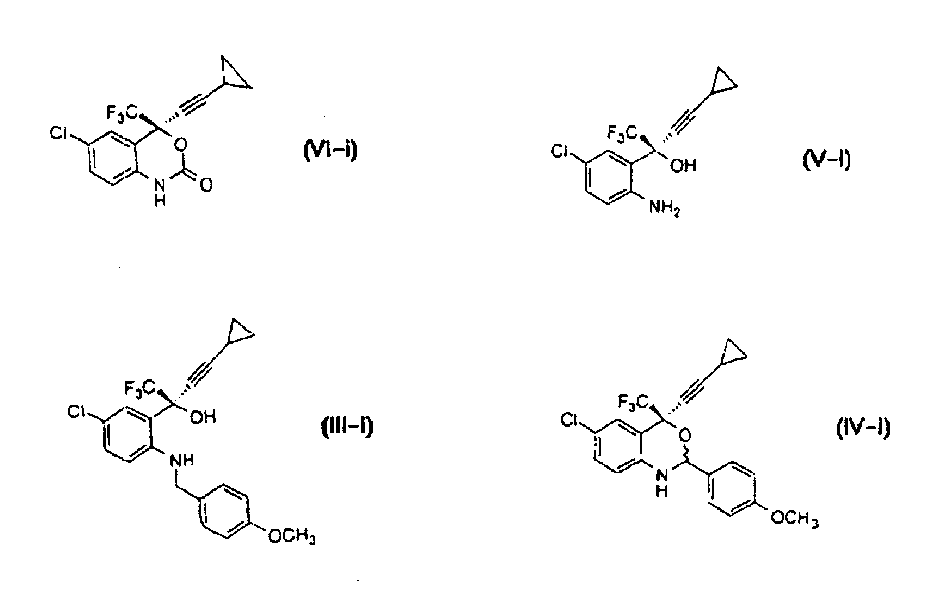
Asymmetric synthesis of benzoxazinones via new intermediates
A compound, aniline technology, applied in the field of human immunodeficiency virus reverse transcriptase inhibitors, can solve problems such as expensive and unstable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0216] Preparation of N-(4-chlorophenyl)-2,2-dimethylpropanamide. 4-Chloroaniline (52.7kg, 413mol) was dissolved in a mixture of tert-butyl methyl ether (180kg), 30% aqueous sodium hydroxide solution (61.6kg, 463mol) and water (24.2kg), then cooled to 15°C. To the resulting slurry was added trimethylacetyl chloride (52.2 kg, 448 mol) over 1 hour keeping the temperature below 40°C. After stirring at 30°C for 30 minutes, the slurry was cooled to -10°C and held for 2 hours. The product was collected by filtration, washed with a 90 / 10 water / methanol solution (175 kg), and dried in vacuo to afford 85 kg (97% yield) of the title compound as a crystalline solid.
[0217] mp152-153°C; 1 H NMR (300MHz, CDCl 3 )δ7.48(d, J=9Hz, 2H)7.28(d, J=9Hz, 2H); 13 C NMR (75MHz, CDCl 3 )d176.7, 136.6, 129.1, 128.9, 121.4, 39.6, 27.6.
[0218] Preparation of N-(4-fluorophenyl)-2,2-dimethylpropanamide. Those skilled in the art of organic synthesis should know that this compound can be e...
Embodiment 2
[0220] Preparation of the hydrochloride hydrate of 4-chloro-2-trifluoroacetylaniline. N-(4-chlorophenyl)-2,2-dimethylpropanamide (36.7kg, 173mol) was added to TMEDA (20.2kg, 174mol) in anhydrous tert-butyl methyl ether (271.5kg) solution, and then Cool to -20°C. To this cold slurry was added 2.7N n-butyllithium in hexane (101.9 kg, 393 mol) while maintaining the temperature below 5°C. After aging at 0-5°C for 2 hours, the solution was cooled to below -15°C and reacted rapidly with ethyl trifluoroacetate (34.5 kg, 243 mol). After 30 minutes, the resulting solution was poured into 3N HCl (196 L, 589 mol) maintaining the temperature below 25 °C. After removing the aqueous phase, the organic solution was concentrated by distilling off approximately 200 L of solvent. Acetic acid (352 kg) was added while 325 kg of solvent was distilled off under 100 mm vacuum. After the solution was cooled to 30°C, 12N HCl (43.4kg, 434mol) was added, and the mixture was heated to 65-70°C for 4 h...
Embodiment 3-a
[0223] Preparation of 4-chloro-2-trifluoroacetylaniline. 4-Chloro-2-trifluoroacetylaniline hydrochloride hydrate (17.1 g, 62 mmol) was stirred in a mixture of toluene (100 ml) and water (50 ml). with saturated NaHCO 3 The solution neutralized the mixture to pH7. The organic phase was then concentrated in vacuo and the residue was recrystallized from heptane to afford 12.5 g (91%) of the title compound as yellow needles: mp 98-99°C.
[0224] 1 H NMR (300MHz, CDCl 3 )δ7.70(t, J=2Hz, 1H), 7.32(dd, J=2, 9Hz, 1H), 6.7(d, J=9Hz, 1H), 6.44(brs, 2H); 13 C NMR (75MHz CDCl 3 )δ180.0, 151.6, 136.9, 130.1, 120.9, 119.0, 116.8, 111.4; 19 F NMR (282 MHz, CDCl 3 )δ-70.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com