Chiral alpha-(trichloromethyl) amine compound and preparation method thereof
An amine compound and trichloromethyl technology are applied in the field of chiral α-amine compounds and their preparation, which can solve the problems that raw material sultam is not easy to prepare, cannot meet the needs of development, and has low optical purity, etc. The effect of industrialized implementation, mild preparation process conditions, and economical and easy-to-obtain raw materials
Inactive Publication Date: 2010-10-13
SHANGHAI UNIV OF ENG SCI
View PDF1 Cites 9 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
In addition, the literature Dilman, A.D.; Arkhipov, D.E.; Levin, V.V.; Belyakov, P.A.; Korlyukov, A.A.; 3 Preparation of racemic α-(trichloromethyl)amine by addition of salicylaldimine, but based on TMSCCl 3 The preparation of chiral α-(trichloromethyl)amine by addition of chiral imine is still a blank
At present, as the above-mentioned document Miltz, W.; Steglich, W.Synthesis 1990, 9, the preparation method reported in 750 needs to utilize expensive chiral enamine: above-mentioned document Zajac, M.; Peters, R.Org.Lett. The method reported in 2007, September, 2007 has the problem that the raw material sultone is not easy to prepare and the optical purity is not high
In addition, the above-mentioned preparation methods all have universal defects and cannot meet the needs of the development of related fields.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
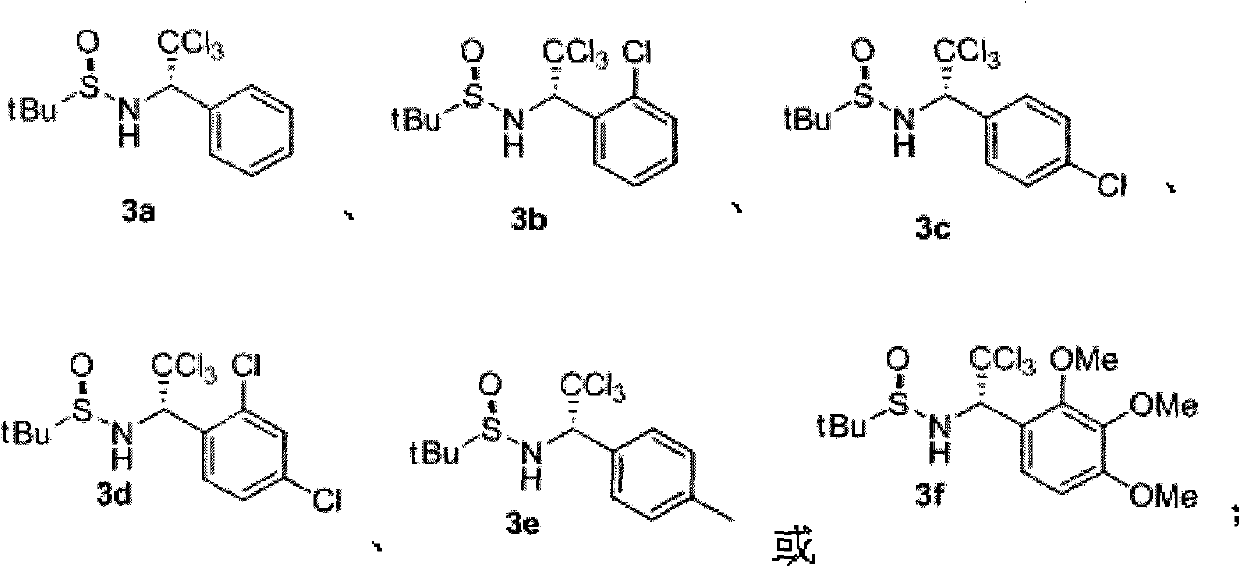
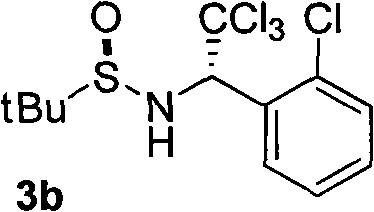
The invention discloses a chiral alpha-(trichloromethyl) amine compound and a preparation method thereof. The chiral alpha-(trichloromethyl) amine compound is a potential bioactive molecule synthesis building block and can serve as an important midbody for synthesizing chiral chloric amine compounds, such as 2,2-dichloro aziridine. The preparation method of the invention has moderate technological condition and abundant and cheap raw materials, and the prepared alpha-(trichloromethyl) amine has high optical purity and is convenient to industrially apply. The alpha-(trichloromethyl) amine prepared by the invention can be widely applied in the fields of asymmetric synthesis and medicine research and development. The structural general formula is disclosed in formula (3).
Description
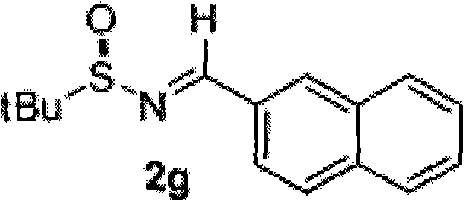
technical field The invention relates to chiral α-(trichloromethyl)amine compounds and a preparation method thereof. Background technique The structural units of α-(trichloromethyl)amine compounds exist in biologically active molecules, see literature: (a) Bringmann, G.; Feineis, D.; God, R.; Maksimenka, K.; Muhlbacher, J.; K. Tetrahedron 2004, 60, 8143; (b) FAHMY, Mohamed, Abdel, Hamid. WO 91 / 12228, 1991.) reports. α-(trichloromethyl)amines are also very useful organic synthons, which can be converted into 2,2-dichloroaziridines (Zaugg, H.E.; Denet, R.W.J.Org.Chem.1971, 36, 1937-1941), α-amino acid (Shinkevich, E.Y.; Novikov, M.S.; Khlebnikov, A.F. Synthesis 2007, 2, 225) and other important organic compounds. Although α-(trichloromethyl)amines have broad application prospects in the field of life sciences, the preparation methods of chiral α-(trichloromethyl)amines are very limited: based on trichloroethyleneimine as a precursor Only two cases of synthesis have ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07C313/06C07B53/00C07D307/52C07D213/42
Inventor 李亚孙智华王晗曹韵律顾佳颍
Owner SHANGHAI UNIV OF ENG SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com