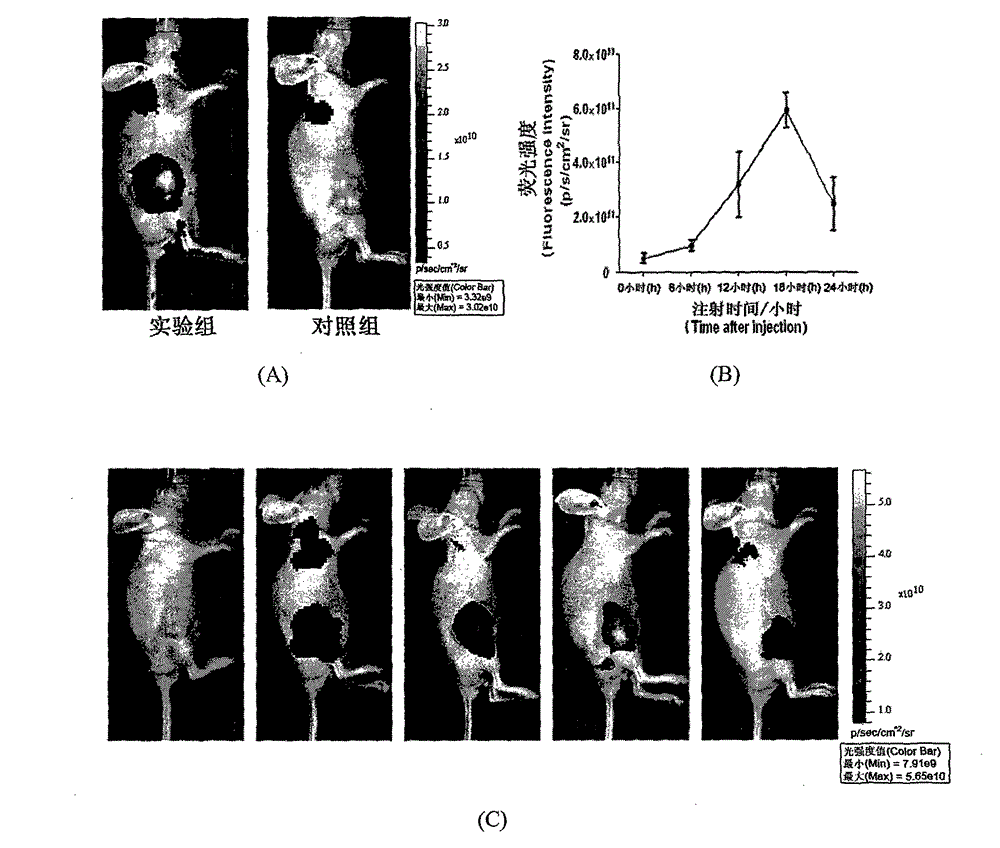
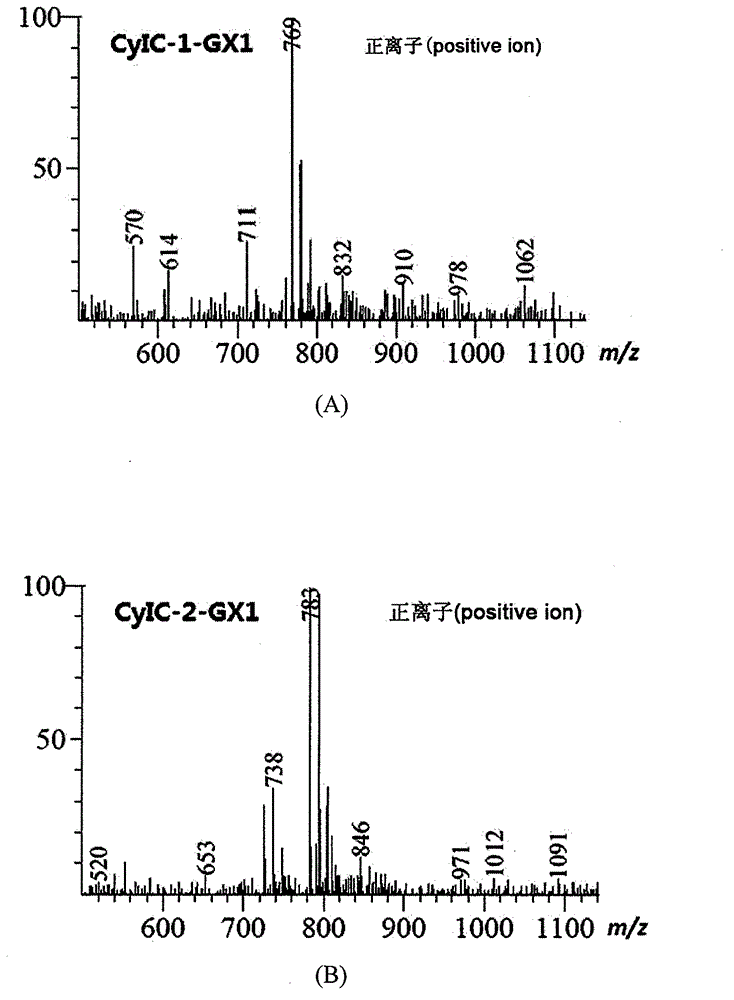
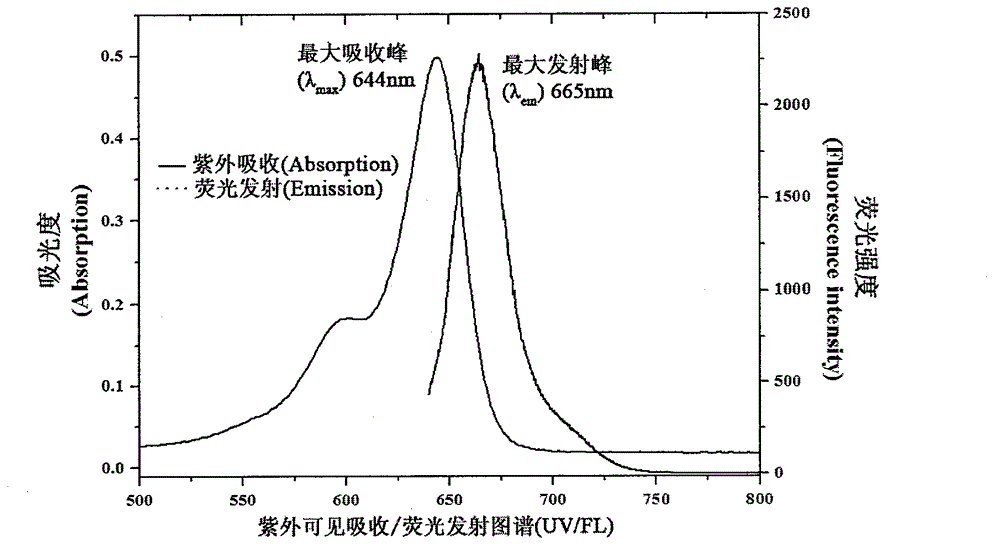
Symmetric pentamethyl cyanine dye and application thereof to molecular imaging
A cyanine dye, symmetrical technology, applied in the field of organic compounds and their preparation, can solve the problems of complex synthetic routes, poor photostability, and many by-products, and achieve strong membrane permeability, simplified synthetic steps, and low toxicity of living cells Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Preparation of Compound B1 and Compound B2:
[0052] (1) Synthesis of iodide N-methyl-2,3,3-trimethyl-5-sulfonic acid group-3H-indole quaternary ammonium salt (compound B1)
[0053] Add 2.8g (10mmol) 2,3,3-trimethyl-5-sulfonic acid-3H-indole A and 2.8g (20mmol) iodomethane into a sealed reaction kettle containing 40mL methanol, and heat to 75°C React for 24 hours; if ethanol is used instead of methanol as the reaction solvent, the reaction temperature is 85°C; if iodomethane is directly used as the reaction solvent, the reaction temperature is 70°C, after cooling, part of the methanol is evaporated in a vacuum rotary until solid appears at the bottom of the bottle, and suction filtered The crude product was washed with ether to obtain 3.3 g of yellow-brown solid quaternary ammonium salt with a yield of 80%.
[0054] (2) Synthesis of iodide N-ethyl-2,3,3-trimethyl-5-sulfonic acid group-3H-indole quaternary ammonium salt (compound B2)
[0055] Add 2.8g (10mmol) 2,3,3-tr...
Embodiment 2
[0057] The preparation of Wujiachuan condensation agent compound C:
[0058] Dissolve 5g (30mmol) of dichlorocrotonin in 15mL of absolute ethanol, add 5.8g (60mmol) of aniline in 20mL of absolute ethanol, heat at 80-85°C for 30min under reflux; or use methanol as the reaction solvent , heated at 70-75° C. to reflux for 30 minutes, an orange solid precipitated out, was filtered by suction, washed with ether, and recrystallized from absolute ethanol to obtain 7.6 g of a bright orange solid with a yield of 86%.
Embodiment 3
[0060] Synthesis of dye CyI-1 and dye CyI-2:
[0061]
[0062] Put 7mmol indole quaternary ammonium salt B1 or B2 and 0.0035mmol pentamethine condensing agent C in 45mL glacial acetic acid and 45mL acetic anhydride, and react under reflux for 1 to 2 hours. The ratio is strictly 2:1. When the molar ratio of feed is less than 2:1, the by-product of pentamethine semicyanine dye will be generated. After the reaction, the crude product was recrystallized with methanol:water=5:1 to obtain a bright dark green solid, and the yield of dye CyI-1 and dye CyI-2 was about 50%. The NMR and mass spectrometry data of product structure identification are as follows:
[0063] CyI-1: 1 H NMR (400 MHz, DMSO-d 6 ) δ (ppm): 1.73 (s, 12H, C (CH 3 ) 2 ), 3.68(s, 6H, NCH 3 ), 6.29 (d, 2H, J=13.6 Hz, -CH=), 7.43 (d, 2H, J=8.0 Hz, ArH), 7.67 (d, 2H, J=8.0 Hz, ArH), 7.88 (s, 2H, ArH), 8.47 (d, 2H, J=13.6 Hz, -CH=). 13 C NMR (400 MHz, DMSO-d 6 )δ (ppm): 26.04, 66.93, 99.72, 110.30, 119.52, 121...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com