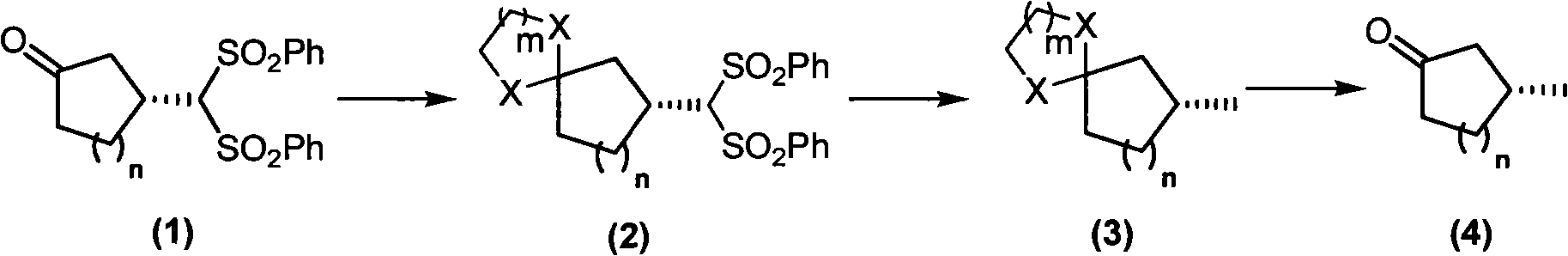
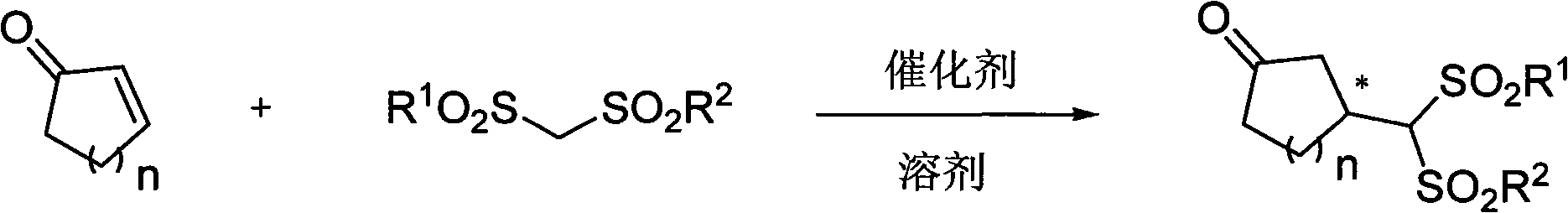Asymmetric synthesis of chiral muskone and other 3-methyl cyclic ketone
A technology of methyl cyclic ketone and muskone, applied in the chemical field, can solve the problems of waste, low yield, low enantioselectivity, etc., and achieve the effects of high conversion rate, good selectivity, and high optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The reaction formula is:
[0057]
[0058] In 0.5 mL of 1,4-dioxane, add bisphenylsulfonylmethane (322 mg, 0.75 mmol), pentadecenone (111 mg, 0.5 mmol), benzoic acid (24.4 mg, 0.2 mmol), catalyst ( 9)(R 5 is methoxy, R 6 Vinyl) (32.3mg, 0.1mmol), placed at 35°C and stirred for 4 days after the feeding was completed. After the reaction conversion was complete, the reaction liquid was concentrated under reduced pressure and separated by silica gel column chromatography (petroleum ether / ethyl acetate=10: 1) 192mg of the addition product was isolated as a white solid with a yield of 74%, a melting point of 142-144°C, and an optical rotation [α] 24 D =-20.0° (c=1.00, CH 2 Cl 2 ); 1 H NMR (400MHz, CDCl 3 ): δ (ppm) 7.94 (dd, J=7.6, 5.2Hz, 4H), 7.71-7.65 (m, 2H), 7.60-7.53 (m, 4H), 4.72 (s, 1H), 3.11-3.06 (m , 1H), 3.02~3.00(m, 2H), 2.52~2.45(m, 1H), 2.30~2.23(m, 1H), 1.87~1.69(m, 3H), 1.36~1.17(m, 19H). 13 CNMR (100MHz, CDCl 3 ): δ (ppm) 209.8, 140.3, 138.3, 134....
Embodiment 2
[0060] The difference from Example 1 is that the cyclic enone used is 3-cyclohexen-2-one, the reaction temperature is 4°C, other experimental methods and conditions are the same as in Example 1, and the addition product of white solid is obtained , the yield is 81%, the melting point is 176~177℃, the optical rotation [α] 23 D =-8.5° (c=1.0, CH 2 Cl 2 ); 1 H NMR (400MHz, CDCl 3 ): δ(ppm) 7.95~7.91(m, 4H), 7.74~7.70(m, 2H), 7.61~7.56(m, 4H), 4.45(s, 1H), 3.19(t, J=13.6Hz, 1H ), 2.79(t, J=12.8Hz, 1H), 2.45~2.28(m, 4H), 2.11~2.06(m, 1H), 1.93(d, J=13.2Hz, 1H), 1.55~1.43(m, 1H). 13 C NMR (100MHz, CDCl 3 ): δ (ppm) 208.3, 139.1, 138.4, 134.7, 134.6, 129.4, 129.2, 86.0, 45.6, 40.8, 38.8, 27.8, 25.0; HRMS (ESI): theoretical value [M+NH 4 ] + (C 19 h 24 NO 5 S 2 ) 410.1096, actually obtained 410.1091; obtain chiral analysis by HPLC, concrete conditions are: [IA column, 220nm, n-Hexane: DCM=2: 1, 0.8mL / min]: 27.5min (primary), 31.7min (secondary ), ee=95%.
Embodiment 3
[0062] The difference from Example 1 is that the cyclic enone used is 3-cyclohepten-2-one, other experimental methods and conditions are the same as in Example 1, and the addition product of a white solid is obtained with a yield of 92%. , melting point 212~214℃; optical rotation [α] 23 D =-43.6° (c=0.50, CH 2 Cl 2 ); 1 H NMR (400MHz, CDCl 3 ): δ(ppm) 7.95~7.92(m, 4H), 7.73~7.69(m, 2H), 7.60~7.56(m, 4H), 4.51(s, 1H), 3.34(dd, J=12, 2.8Hz , 1H), 2.86(t, J=11.2Hz, 1H), 2.57~2.41(m, 3H), 2.23~2.10(m, 2H), 1.68~1.59(m, 1H), 1.33~1.24(m, 2H ), 0.90~0.85 (m, 1H). 13 C NMR (100MHz, CDCl 3 ): δ (ppm) 211.3, 138.9, 138.8, 134.7, 134.6, 129.5, 129.4, 129.2, 88.3, 47.3, 43.6, 37.1, 34.2, 29.4, 23.7. HRMS(ESI): theoretical value [M+NH 4 ] + (C 20 h 26 NO 5 S 2 ) is 424.1252, and the actual result is 424.1250; chiral analysis is obtained by HPLC, and the specific conditions are: [IA column, 220nm, Hexane: DCM=2: 1, 0.8mL / min]: 20.9min (time), 24.3min (main) , ee=92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com