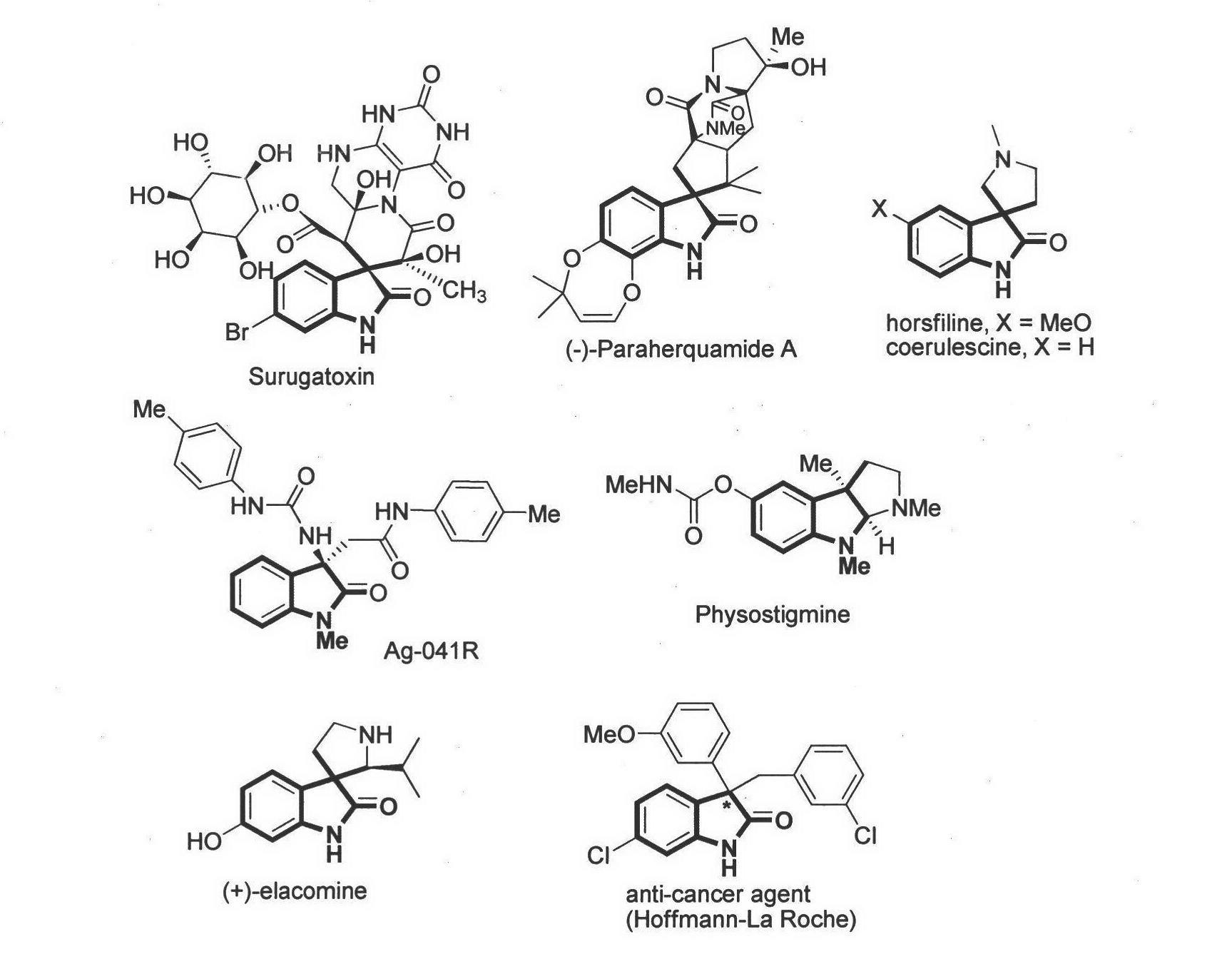
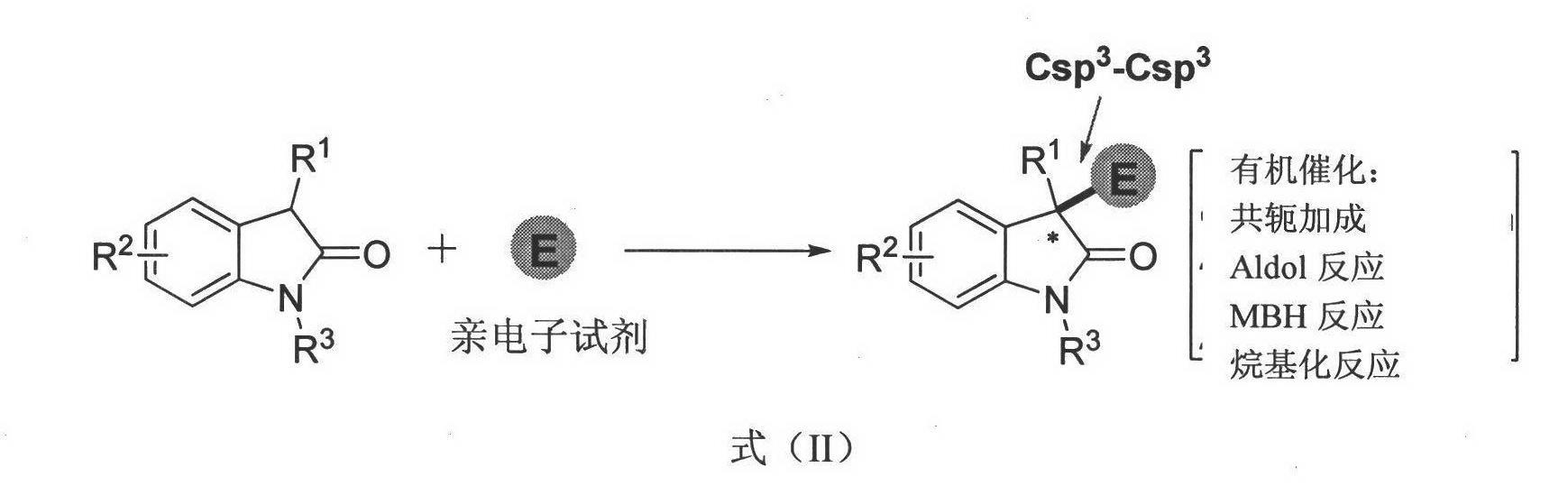
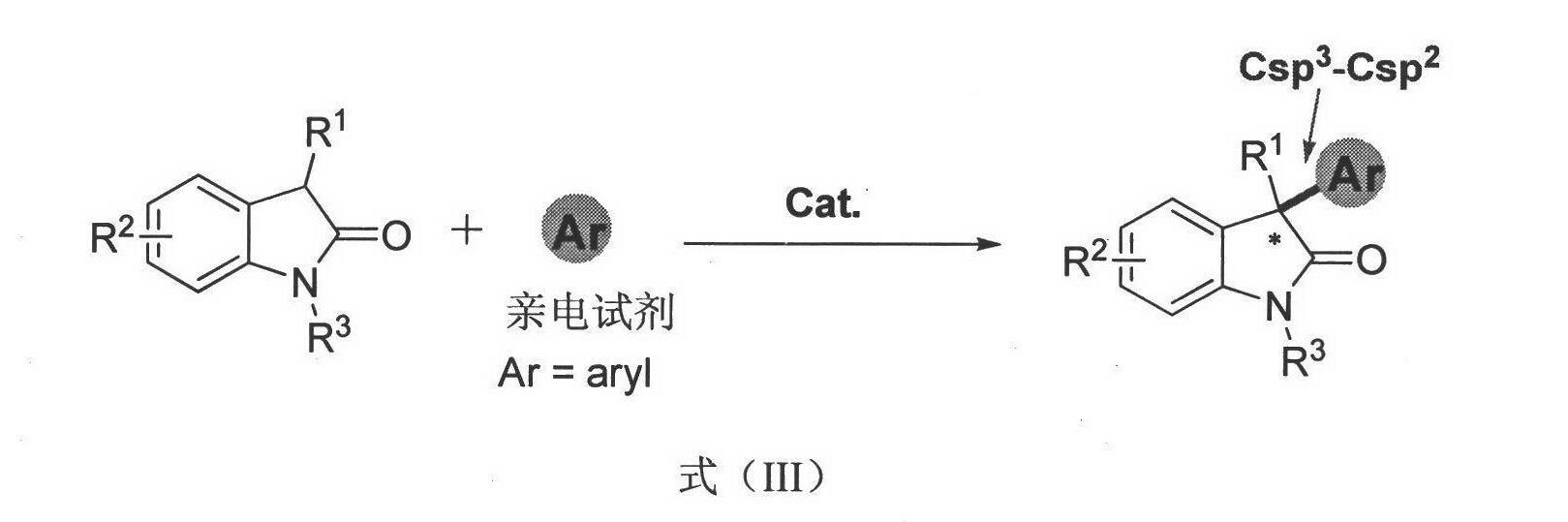
Method for asymmetric synthesis of 3,3-disubstituted-2-oxindole compound
A technology of indole compound and oxindole, applied in the direction of asymmetric synthesis, organic chemical methods, chemical instruments and methods, etc., to achieve the effect of low price, wide sources and less pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
[0027] In reaction vial A, substrate 2a (16 mg, 0.10 mmol) was dissolved in 0.1 mL DCM and stirred for 10 min. Meanwhile, in Reaction Vial B, Oxindole Substrate 1 (37 mg, 0.12 mmol) and Catalyst I (4.1 mg, 0.01 mmol) were dissolved in 0.5 mL DCM and stirred at room temperature. Then, the reaction liquid in the reaction bottle A was added dropwise into the reaction bottle B, and reacted for 3 hours. The progress of the reaction was detected by TLC. After the reaction was completed, water was added and extracted with ethyl acetate. The organic phases were combined, washed once with water and once with saturated brine, and dried over anhydrous magnesium sulfate. Finally, the target product 3a was purified and isolated by flash column chromatography, 38.6 mg, with a yield of 83% and ee=64%. 1 H NMR (500MHz, CDCl 3 ): δ8.04(dd, J=1.5, 7.5Hz, 1H), 8.00(d, J=8.0Hz, 1H), 7.96(dd, J=1.5, 7.5Hz, 1H), 7.74-7.67(m, 2H), 7.43-7.36(m, 6H), 7.19(m, 1H), 7.13(dd, J=1.0, 7.5Hz,...
Embodiment 2
[0029]
[0030] In reaction vial A, substrate 2a (16 mg, 0.10 mmol) was dissolved in 0.1 mL of DCM and stirred for 10 minutes. Meanwhile, in reaction vial B, oxindole substrate 1 (37 mg, 0.12 mmol) and catalyst II (6.0 mg, 0.01 mmol) were dissolved in 0.5 mL of DCM and stirred at room temperature. Then, the reaction liquid in the reaction bottle A was added dropwise into the reaction bottle B, and reacted for 3 hours. The progress of the reaction was detected by TLC. After the reaction was completed, water was added and extracted with ethyl acetate. The organic phases were combined, washed once with water and once with saturated brine, and dried over anhydrous magnesium sulfate. Finally, the target product 3a was purified and isolated by flash column chromatography, 30 mg, with a yield of 65% and ee=62%. The product was detected as compound 3a by spectrogram.
Embodiment 3
[0032]
[0033] In reaction vial A, substrate 2a (16 mg, 0.10 mmol) was dissolved in 0.1 mL DCM and stirred for 10 min. Meanwhile, in reaction vial B, oxindole substrate 1 (37 mg, 0.12 mmol) and catalyst III (4.9 mg, 0.01 mmol) were dissolved in 0.5 mL DCM and stirred at room temperature. Then, the reaction liquid in the reaction bottle A was added dropwise into the reaction bottle B, and reacted for 3 hours. The progress of the reaction was detected by TLC. After the reaction was completed, water was added and extracted with ethyl acetate. The organic phases were combined, washed once with water and once with saturated brine, and dried over anhydrous magnesium sulfate. Finally, the target product 3a was purified and isolated by flash column chromatography, 39.5 mg in yellow solid, with a yield of 85%, ee=75%. The product was detected as compound 3a by spectrogram.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com